The Smallest Artificial Pancreas System Receives FDA Clearance - JDRF
4.6 (195) In stock
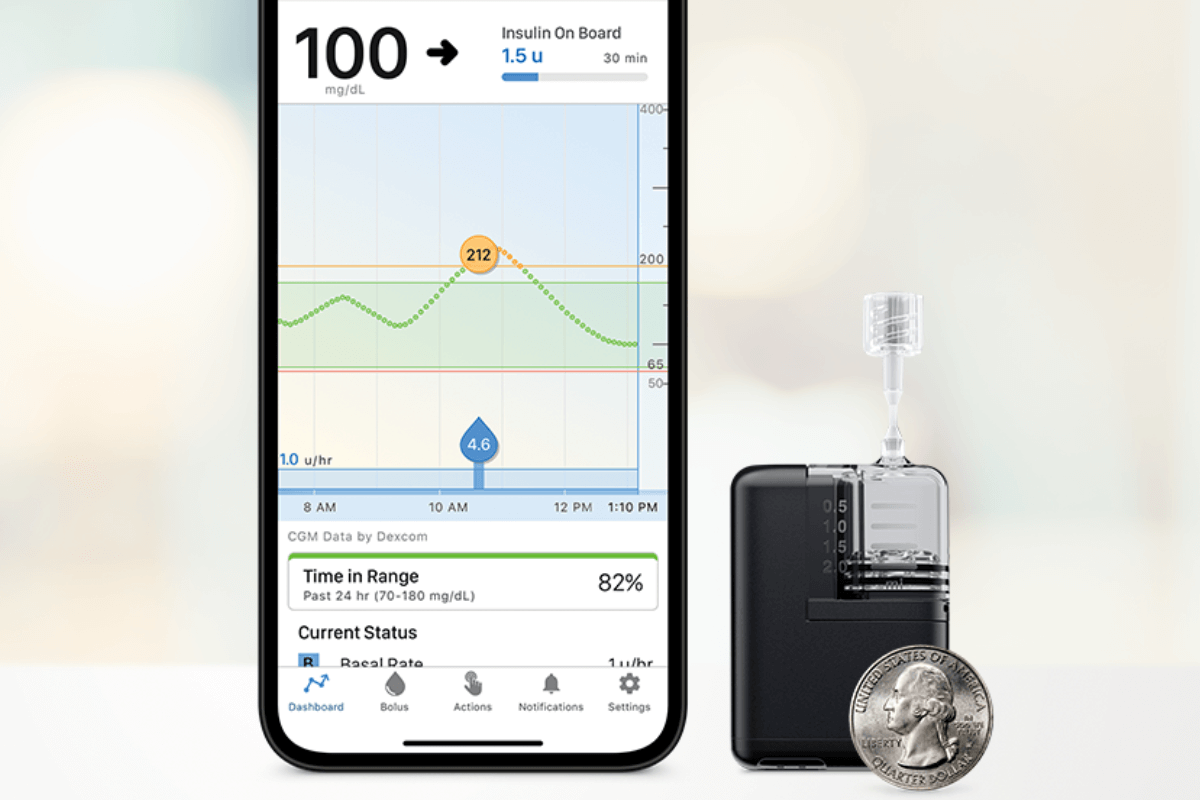
In just the past year, we have had multiple artificial pancreas systems authorized by the Food and Drug Administration (FDA)…and it’s not stopping! Last week, Tandem Mobi—a miniature-sized insulin pump, for use with Tandem’s Control-IQ™ technology and a compatible continuous glucose monitor (CGM)—received FDA clearance. The Tandem Mobi is half the size of the company’s…
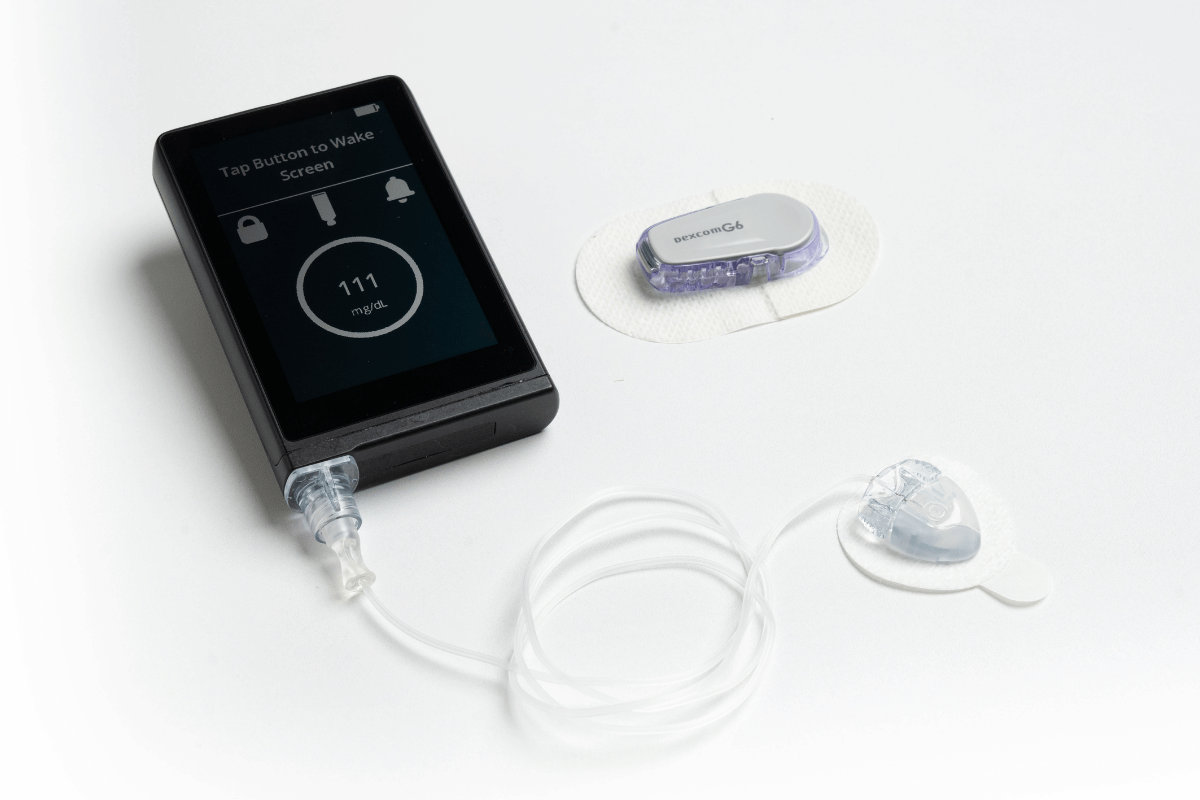
FDA Cleared a New Artificial Pancreas System: JDRF Report

Do-It-Yourself (DIY) Artificial Pancreas Systems for Type 1

Control IQ Gets FDA Approval: All About Tandem's Hybrid Closed

FDA approves first artificial pancreas for type 1 diabetes, Medtronic's MiniMed 670G - CBS News

For People With Type 1 Diabetes, An 'Artificial Pancreas' Is
Racing for a Cure: How the Artificial Pancreas Helped One Man

Artificial Pancreas: Current Progress and Future Outlook in the

Automated closed-loop control of diabetes: the artificial pancreas
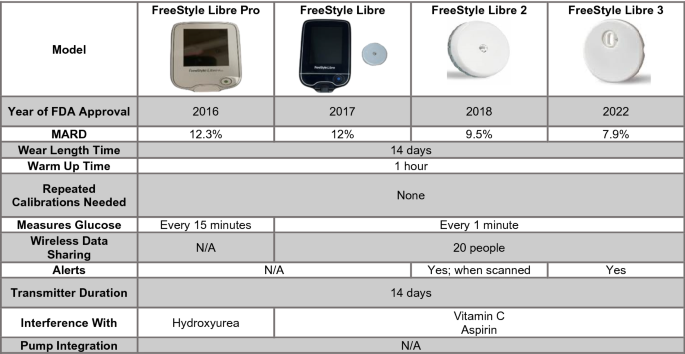
Emerging Diabetes Technologies: Continuous Glucose Monitors

i0.wp.com/post./wp-content/uploads/2
Hackers Made an App That Kills to Prove a Point
Insulin Pump - a beacon of hope for children with Type 1 Diabetes
FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice
Insider's Guide: Latest Insulin Pump Advances! - Let's change diabetes together
 Mountain Equipment, Pants, Mountain Equipment Coop Istrum Pants Mens 38x34 Hiking Outdoor Stretch Blue Mec
Mountain Equipment, Pants, Mountain Equipment Coop Istrum Pants Mens 38x34 Hiking Outdoor Stretch Blue Mec DECATHLON DOMYOS High-Waisted Body-Sculpting Fitness Leggings
DECATHLON DOMYOS High-Waisted Body-Sculpting Fitness Leggings Women's Exotic Teddies & Bodysuits - Women's Exotic Teddies & Bodysuits / Women': Clothing, Shoes & Jewelry
Women's Exotic Teddies & Bodysuits - Women's Exotic Teddies & Bodysuits / Women': Clothing, Shoes & Jewelry Spanx Moto Faux Leather Leggings International Society of Precision Agriculture
Spanx Moto Faux Leather Leggings International Society of Precision Agriculture adidas Performance Essentials Stripe Sports Tank Top
adidas Performance Essentials Stripe Sports Tank Top Mens Winter Fleece Lined 100% Cotton Thermal Long Johns Top Bottom
Mens Winter Fleece Lined 100% Cotton Thermal Long Johns Top Bottom