Measurement properties of the Swedish clinical outcomes in routine evaluation outcome measures (CORE-OM): Rasch analysis and short version for depressed and anxious out-patients in a multicultural area
4.5 (720) In stock
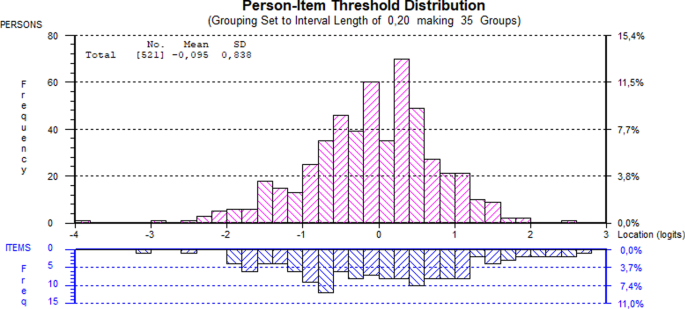
Introduction The Swedish version of the patient-reported Clinical Outcomes in Routine Evaluation Outcome Measures (CORE-OM) has demonstrated high reliability and acceptable convergent validity in explanatory factor analyses. However, the fundamental scale properties have not yet been validated according to item response theory. The aim of this study was to analyze the measurement properties of the Swedish CORE-OM in a cohort of psychiatric out-patients with depression and anxiety in a multicultural area and to explore combinations of items based on shorter versions of the scale (CORE-10, CORE-6D) to improve measurement properties. Methods Data from CORE-OM assessments of 337 patients were analyzed using Rasch analysis. The patients had a mean age of 30 ± 14 years, the majority were women (72%). Requirements for measurement properties were checked: overall model fit, item fit residuals, targeting, internal consistency, differential item functioning and thresholds. Sensitivity to change was also analyzed. Results The CORE-OM showed high internal consistency (person separation index = 0.947) and adequate targeting, but there was overall model misfit (item trait interaction χ2 = 917.53, p < 0.001), indication of local dependency, and differential item functioning in 9 items. The risk items showed problems with disordered thresholds. The emotional component of the shorter CORE-6D showed the best fit for our sample. Adding 3 items to include depressive and trauma-related content resulted in a unidimensional 8-item set with acceptable reliability, model fit, targeting and sensitivity to change. Conclusion For out-patients with diagnosed depression or anxiety in a multicultural area, the Swedish CORE-OM showed high internal consistency, but also validity problems. Based on the shorter CORE-6D version, a unidimensional 8-item set could be an alternative brief measure of psychological distress for this population, but further validity studies are required. Qualitative studies exploring the CORE-OM items in non-native speakers are also warranted.

PDF) Measurement properties of the Swedish clinical outcomes in routine evaluation outcome measures (CORE-OM): Rasch analysis and short version for depressed and anxious out-patients in a multicultural area
PDF) Measurement properties of the Swedish clinical outcomes in routine evaluation outcome measures (CORE-OM): Rasch analysis and short version for depressed and anxious out-patients in a multicultural area

Cross-Culturally Adapted Versions of Patient Reported Outcome Measures for the Lower Extremity

Reference values for generic instruments used in routine outcome monitoring: the leiden routine outcome monitoring study, BMC Psychiatry

PDF) Measurement properties of the Swedish clinical outcomes in routine evaluation outcome measures (CORE-OM): Rasch analysis and short version for depressed and anxious out-patients in a multicultural area

Paula Kramer (Editor) - Namrata Grampurohit (Editor) - Hinojosa and Kramer's Evaluation in Occupational Therapy - Obtaining and Interpreting Data. (2020)

PDF) Psychometric properties of Clinical Outcomes in Routine Evaluation-Outcome Measure (CORE-OM) in Colombia and Peru
IJERPH, Free Full-Text

Abstracts - 2024 - Movement Disorders Clinical Practice - Wiley Online Library
CORE-OM: practitioner overview - Therapy Meets Numbers
 Wooden fishing boats print half sleeve cotton shirt
Wooden fishing boats print half sleeve cotton shirt- Você sabe como o chaves chegou na vila #historia #cultura #chavessuatu
 Buy Triumph Ladyform Soft Minimizer bra smooth skin from £38.56
Buy Triumph Ladyform Soft Minimizer bra smooth skin from £38.56 Summer Savings! Zpanxa Slippers for Women Summer Casual Comfortable Slippers Platform Flip Flops Slippers Flip Flops for Women Brown 40
Summer Savings! Zpanxa Slippers for Women Summer Casual Comfortable Slippers Platform Flip Flops Slippers Flip Flops for Women Brown 40- Not Your Average Cup of Joe
 Dom Casmurro (Ateliê Editorial)
Dom Casmurro (Ateliê Editorial)

