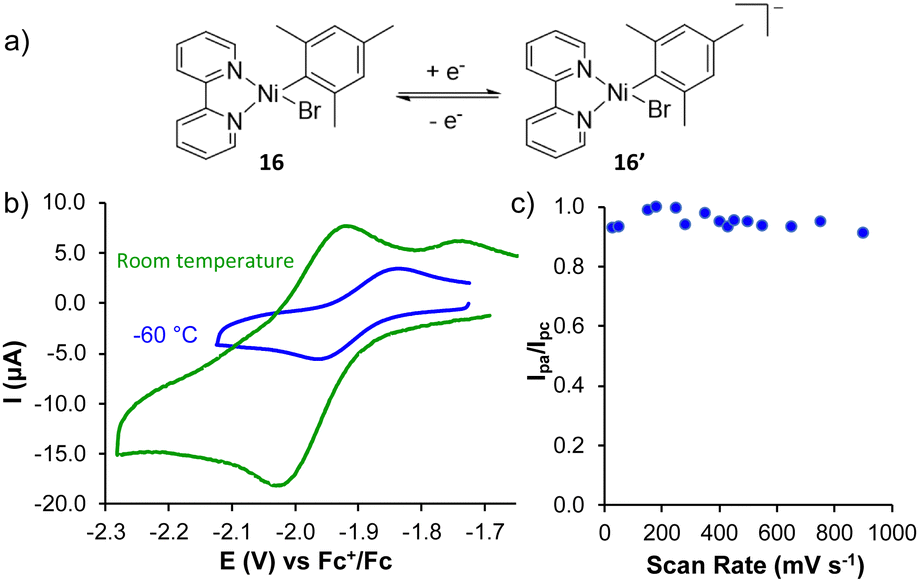
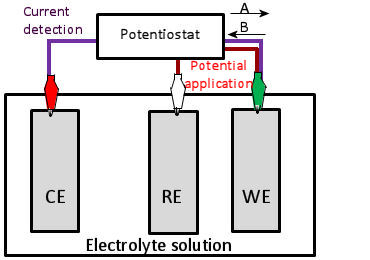
The Cottrell Experiment and Diffusion Limitation 3/3
4.6 (720) In stock

In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.
Cyclic voltammetry and chronoamperometry: mechanistic tools for organic electrosynthesis - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00706A
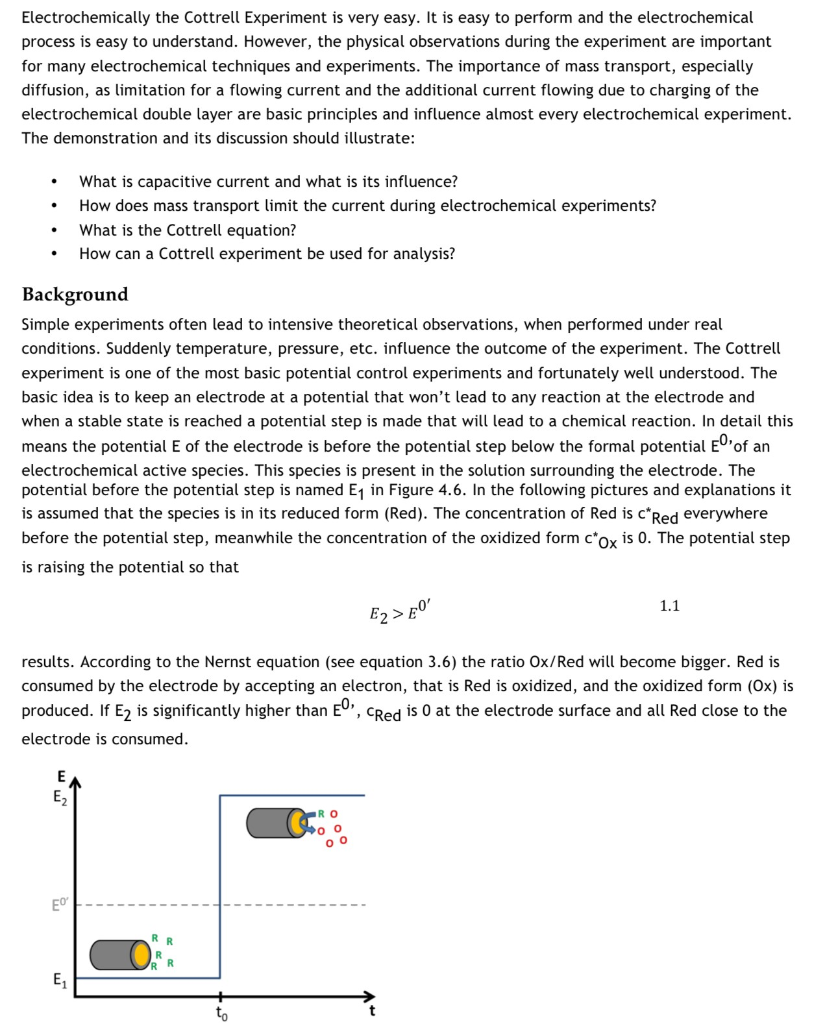
Figure 1.1: Cottrell experiment in KCl solution with

Chapter 3 transport phenomena in electrolytic systems and concentration overpotential. - ppt video online download
support/electrochemical technique

Basic potential step and sweep methods

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes

Fabrication of Ag@Co-Al Layered Double Hydroxides Reinforced poly(o-phenylenediamine) Nanohybrid for Efficient Electrochemical Detection of 4-Nitrophenol, 2,4-Dinitrophenol and Uric acid at Nano Molar Level

PDF) Time of Flight Electrochemistry: Diffusion Coefficient Measurements Using Interdigitated Array (IDA) Electrodes
Theory - Chemistry LibreTexts

5 Mass transport (*diffusion, Fick's laws, Cottrell equation, Nernst diffusion layer)

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes

Slow scan cyclic voltammetry (SSCV) recorded on TLC between ± 1 V with
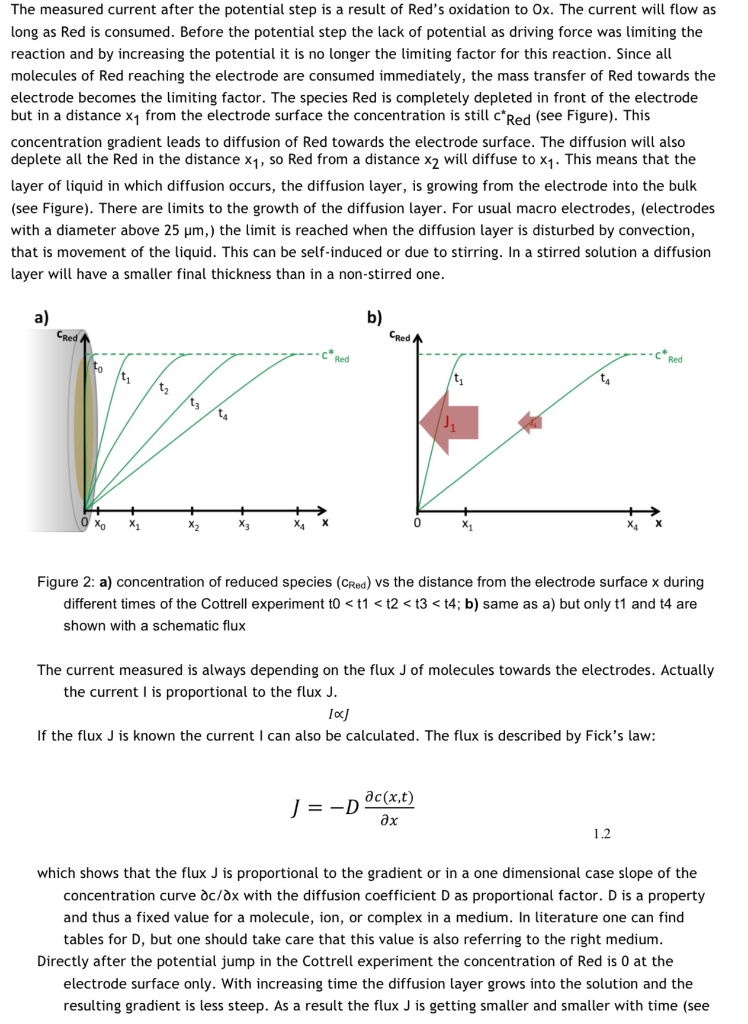
Figure 1.1: Cottrell experiment in KCl solution with
Electrochemical Double Layer - an overview
The electrical double layer (EDL). The EDL can be divided in a Stern
Electrical Double Layer based devices
Principles of EDLCs|TDK Techno Magazine|Electronics ABC|Learn
 Wholesale womes bra For Supportive Underwear
Wholesale womes bra For Supportive Underwear How to Sculpt a Female Face in Zbrush
How to Sculpt a Female Face in Zbrush- Basic White Tank Top Cropped
 White Bra Top and Panties, White Lace Crop Top, Bachelorette Party Lingerie, Sexy Wedding Underwear, No Wire Soft Bra, Longline Bralette - Canada
White Bra Top and Panties, White Lace Crop Top, Bachelorette Party Lingerie, Sexy Wedding Underwear, No Wire Soft Bra, Longline Bralette - Canada Storia lace trim dressy cami top Green Size M - $18 (40% Off
Storia lace trim dressy cami top Green Size M - $18 (40% Off Woods Men's Warden Convertible Pants
Woods Men's Warden Convertible Pants
