For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may
4.5 (737) In stock


Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor
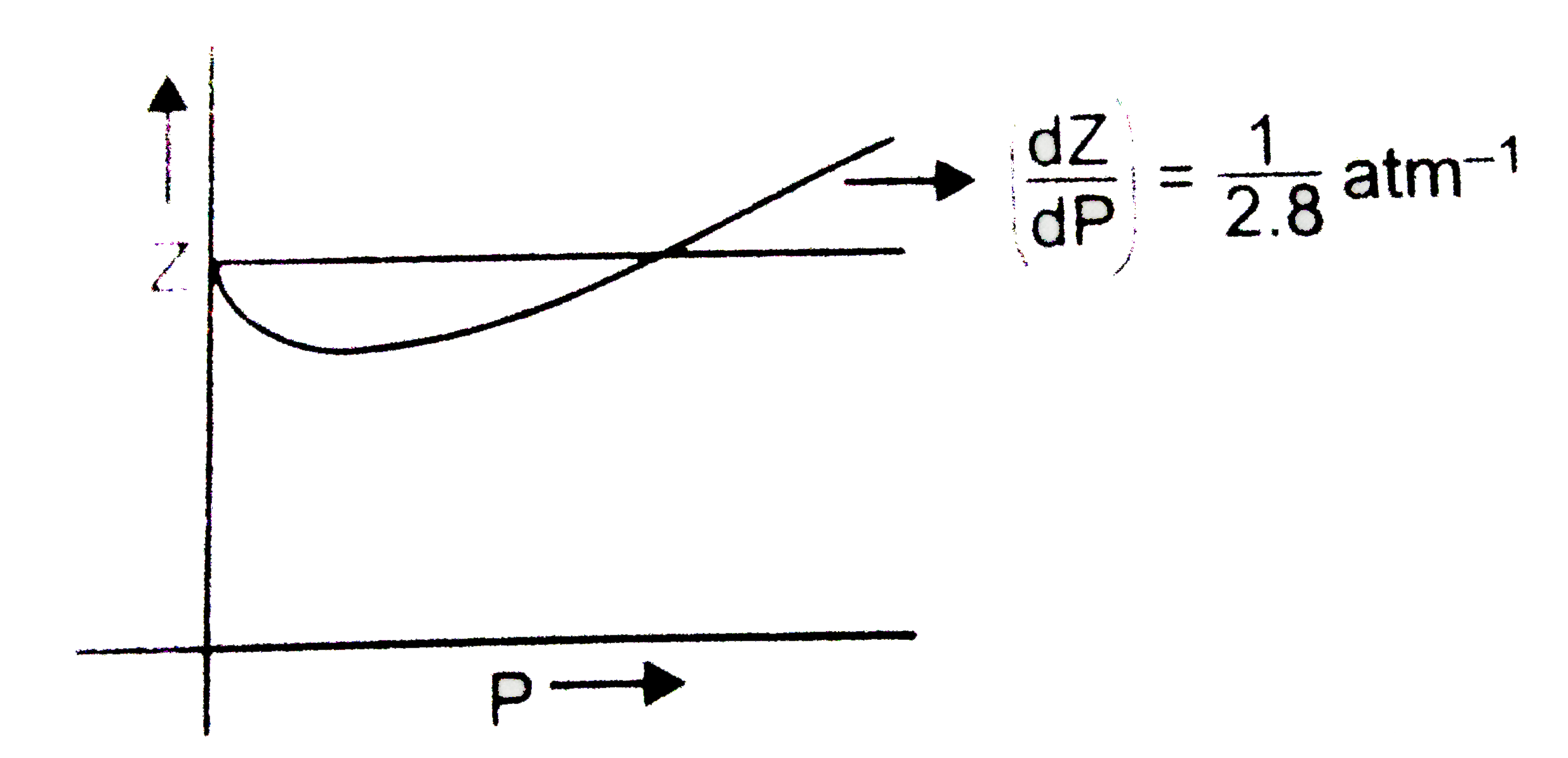
The graph of compressibility factor (Z) vs. P for one mole of a real g

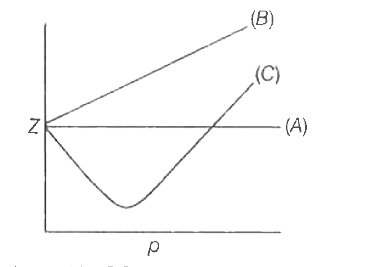
The sketch shows the plot of Z vs P for 1 mole of a hypothetical gas at three distinct temperatures:Boyle's temperature is the temperature at which a gas shows ideal behaviour overpressure
Telugu] The variation of compressibility factor (Z) with pressure (p

Temperature and Pressure Measurements of an Ideal Gas - Because the ideal gas was in a closed - Studocu
Which of the following options will have compressibility factor greater factor greater then 1?H_{2} gas it critical condition.CH_{4} gas room temperature and low pressureN_{4} gas its Boyle's temperature and low pressurehe gas

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?
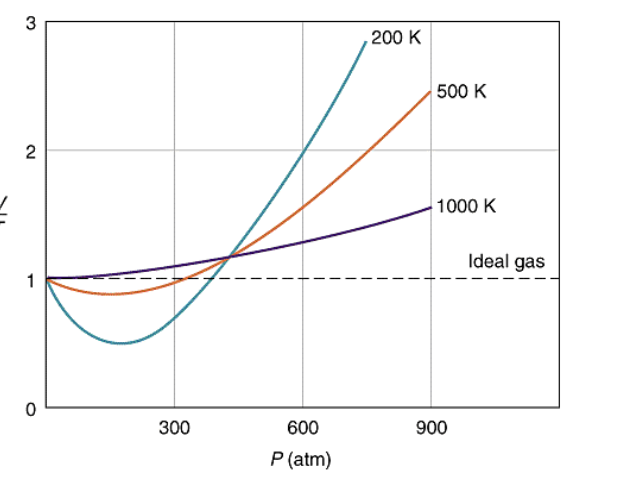
Solved 1. The plot below shows how compressibility factor

ReasonAll the gases tend to approach a value Z=1, when the pressure of gas approaches to zero value any temperature.AssertionAt Boyle temperature, the compressibility factor of a real gas, Zgeq 1.

Compressibility factor - Wikipedia

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.
2024 Significance of compressibility factor - 1. What is meant by
For $CO$, isotherm is of the type as shown. Near the point
Compressibility factor Z for sub-critical pressures in a 'one-cell' formula for excel spreadsheets
 Bell Bottoms With A Flare, Country Music Singer Lainey Wilson Opens Up About Her Retro Western Style And Her Tenacity To Be An Artist
Bell Bottoms With A Flare, Country Music Singer Lainey Wilson Opens Up About Her Retro Western Style And Her Tenacity To Be An Artist Pin by Diana on Moda Denim joggers outfit, Joggers outfit
Pin by Diana on Moda Denim joggers outfit, Joggers outfit- Check Out This Trendy Curvy Queen - Fashion - Nigeria
 Tres tipos de pantalones en los que merece la pena invertir porque
Tres tipos de pantalones en los que merece la pena invertir porque Xlwsbcr Sculpt Peach Women's Fitness Sexy Back Cross Strappy Workout Gym Bra Padded Front Twist Sport Bras Yoga Crop Tank Top - AliExpress
Xlwsbcr Sculpt Peach Women's Fitness Sexy Back Cross Strappy Workout Gym Bra Padded Front Twist Sport Bras Yoga Crop Tank Top - AliExpress Matching ribbed sweatshirt and leggings - Sweatshirts - CLOTHING
Matching ribbed sweatshirt and leggings - Sweatshirts - CLOTHING