Medtronic recalls some insulin pumps that could lead to dangerous incorrect dosing
4.6 (569) In stock
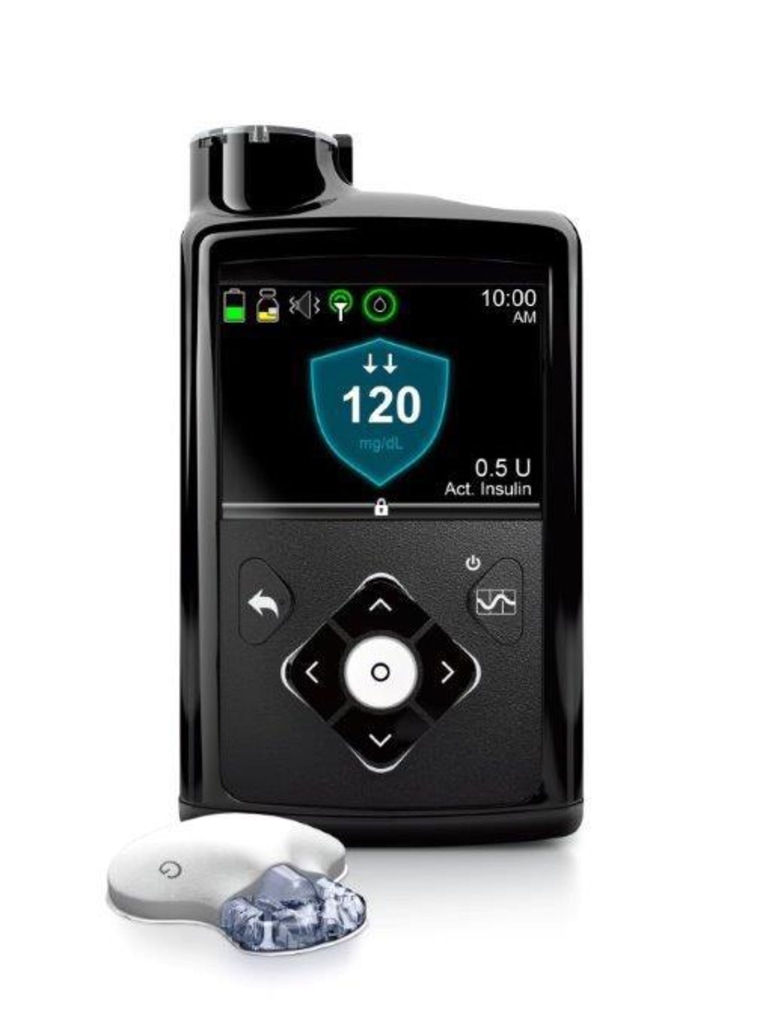
Medtronic is recalling MiniMed 600 Series Insulin Pumps. The FDA calls recall Class I the most serious type of recall, which can lead to injury or death.
This is additional taxonomy that helps us with analytics

Medtronic Issues Urgent Recall of Insulin Pump Controller Vulnerable to Hacks

Medtronic insulin pump recall: FDA says hackers could hijack device - CBS News

FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice

Medtronic Recalls Thousands of Insulin Pumps Due to Dangers

Medtronic Insulin Pump Litigation

Over 300,000 insulin pumps recalled for incorrect dosing issue

Medtronic Recalls MiniMed Insulin Pumps for Incorrect Dosing

Report: Insulin Pumps Have Most Reported Problems In FDA Database - CBS Sacramento

Medtronic Recalls Insulin Pumps Over Concerns For Hacking Risk - CBS Minnesota

Arkansas Medtronic Insulin Pump Lawsuit

Medtronic Issues Urgent Recall of Insulin Pump Controller Vulnerable to Hacks

Medtronic MiniMed Insulin Pump Lawsuit March 2024 - Select Justice

Medtronic Announces FDA Approval for MiniMed 770G System

Insulin pumps linked to more reports of injury and death than any other medical device, records show

Medtronic recalls some insulin pumps as FDA warns they can be hacked
insulin pump diagram – NIH Director's Blog
FDA approves 1st automated, tubeless insulin pump for people with
Insulin Pump Therapy: How It Works and Its Advantages - HealthXchange
- K-12 Blended E-Learning Market Size In 2024 : Forecasting Share
 Women's lingerie online - Swimwear & Homewear
Women's lingerie online - Swimwear & Homewear Front Closure Women Bras Suit Push Up Bra Set Brassiere Sexy Lingerie Briefs BH
Front Closure Women Bras Suit Push Up Bra Set Brassiere Sexy Lingerie Briefs BH Smart & Sexy Women's Plus Size Signature Lace Unlined Underwire Bra with Added Support, No No Red, 40DD
Smart & Sexy Women's Plus Size Signature Lace Unlined Underwire Bra with Added Support, No No Red, 40DD Hooters Racing PSD Boy Shorts Underwear
Hooters Racing PSD Boy Shorts Underwear Teenage Girl Denim Jumpsuits Long Sleeve 2023 Spring Kids Girl Fashion Loose Overalls children clothes 10 12 14 15 Years - AliExpress
Teenage Girl Denim Jumpsuits Long Sleeve 2023 Spring Kids Girl Fashion Loose Overalls children clothes 10 12 14 15 Years - AliExpress
