Microbiological Media Management - SOP & Guideline - Pharma Beginners
4.7 (588) In stock

Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.

SOP of Media Preparation, PDF, Growth Medium
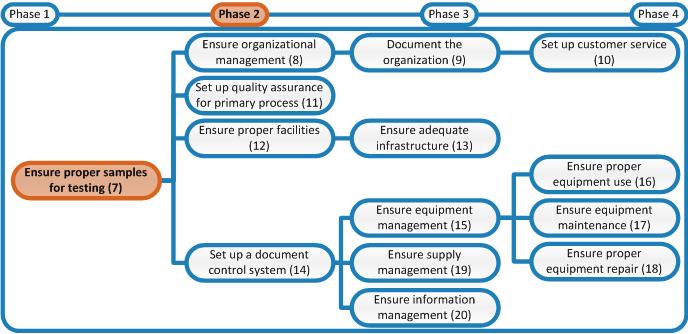
Laboratory Quality Stepwise Implementation tool

Maximizing gained benefits from Standard Operating Procedures
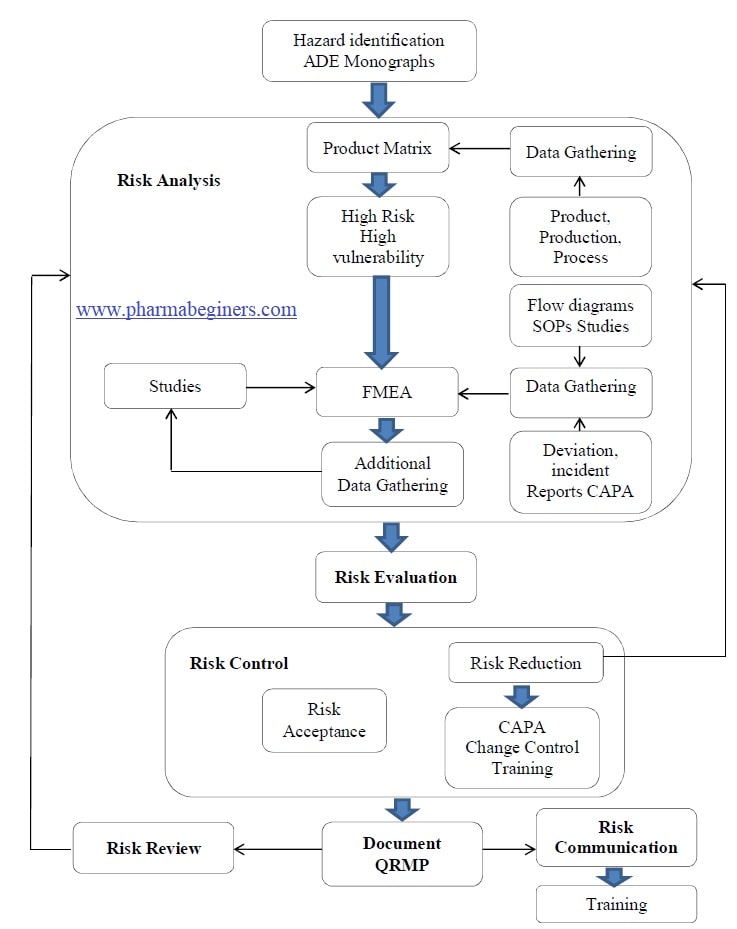
Cross Contamination, Mix-Ups & Microbial Contamination - SOP in Pharma

Suitability of Microbial Count Method & its SOP
The importance of growth promotion testing

Best Microbiology Laboratory Techniques in Pharmaceuticals

Subculturing (Cell Passaging) in Microbiology Lab - Guidelines - SOPs
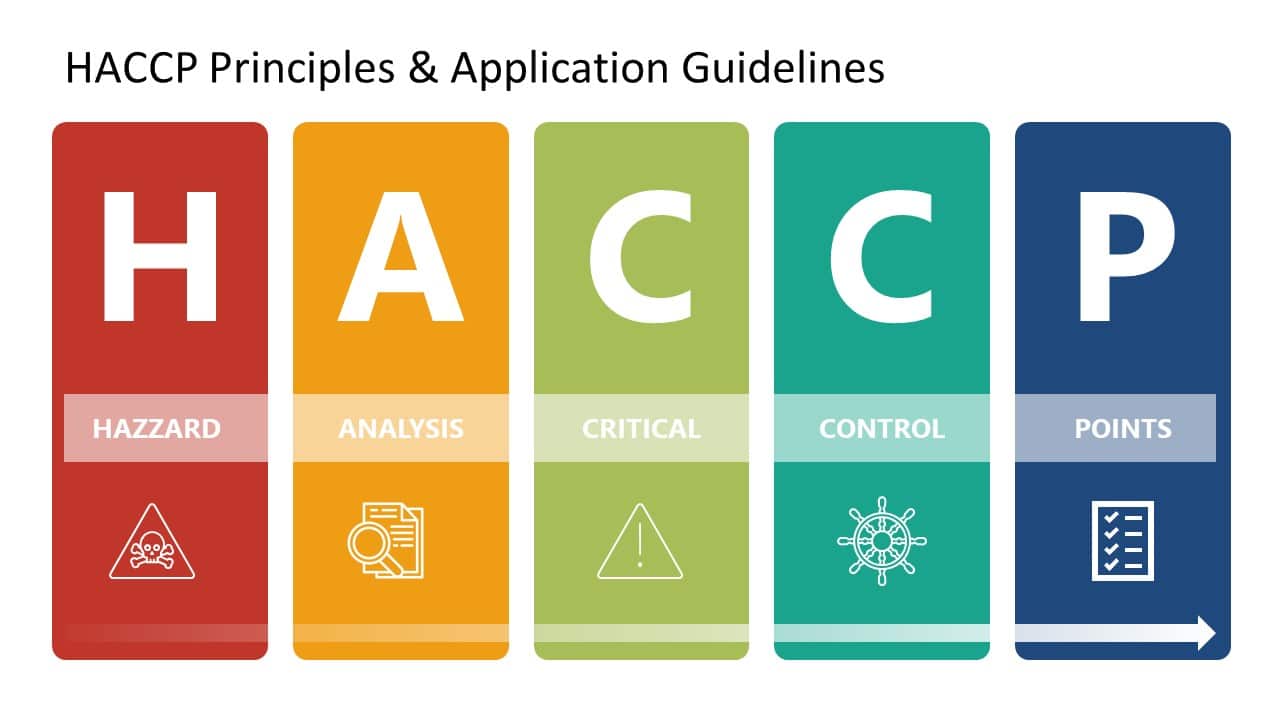
FDA HACCP Principles & Application Guidelines

Microbiology Quality Control Testing: Definition & Procedures
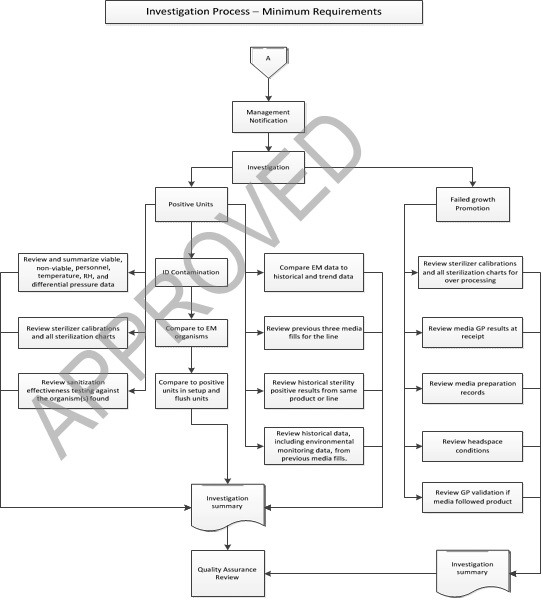
Media Fill Test - Aseptic Process Simulation in Micro - Pharma Beginners

Resume Advice Appreciated : r/biotech

How To Create Great Standard Operating Procedures (9 Steps) - Tettra
Agar Medium Broth Microbial Test Incubate Stock Photo 1012858885
 What causes the sound of thunder?
What causes the sound of thunder? The 12 Best Winter Hiking Pants for Women Winter hiking, Pants for women, Hiking pants
The 12 Best Winter Hiking Pants for Women Winter hiking, Pants for women, Hiking pants- Do girls wear leggings without underwear, and how does it feel? - Quora
 Must-Have Jackets To Wear This Fall
Must-Have Jackets To Wear This Fall Polo Ralph Lauren Men's Short Sleeve Pima Soft Touch Classic Fit Polo, Men's Casual & Dress Polos
Polo Ralph Lauren Men's Short Sleeve Pima Soft Touch Classic Fit Polo, Men's Casual & Dress Polos Royce Comfi Cotton Front Fastening Bra - White Available at The Fitting Room
Royce Comfi Cotton Front Fastening Bra - White Available at The Fitting Room