Solved An ideal gas initially at Pi, V;, and T; is taken
4.6 (764) In stock
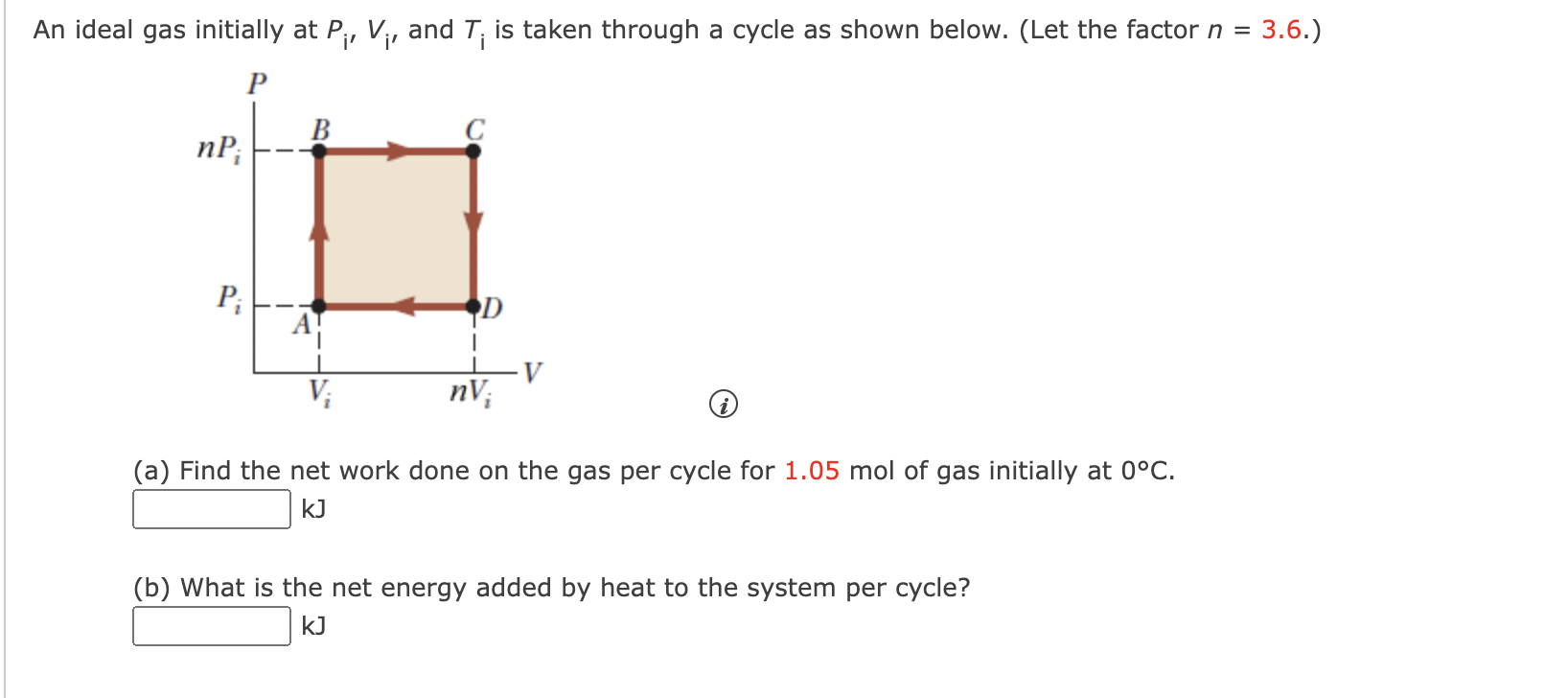
Solved An ideal gas initially at P, V, and T is taken
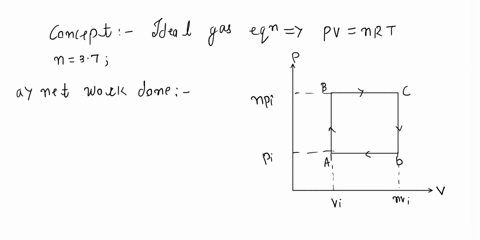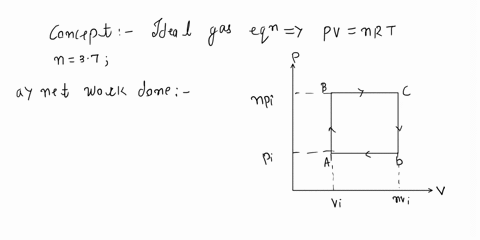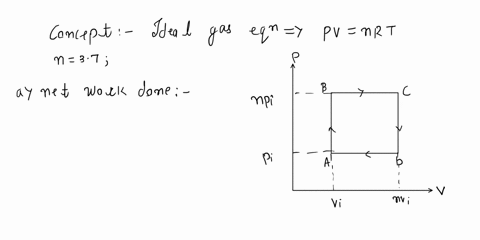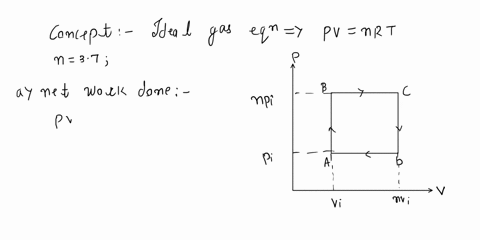
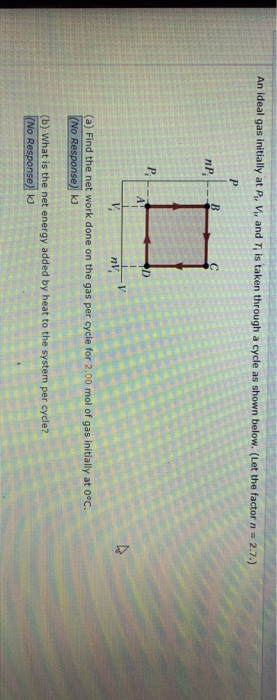
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n = 3.8.) (a) Find the net work done on the
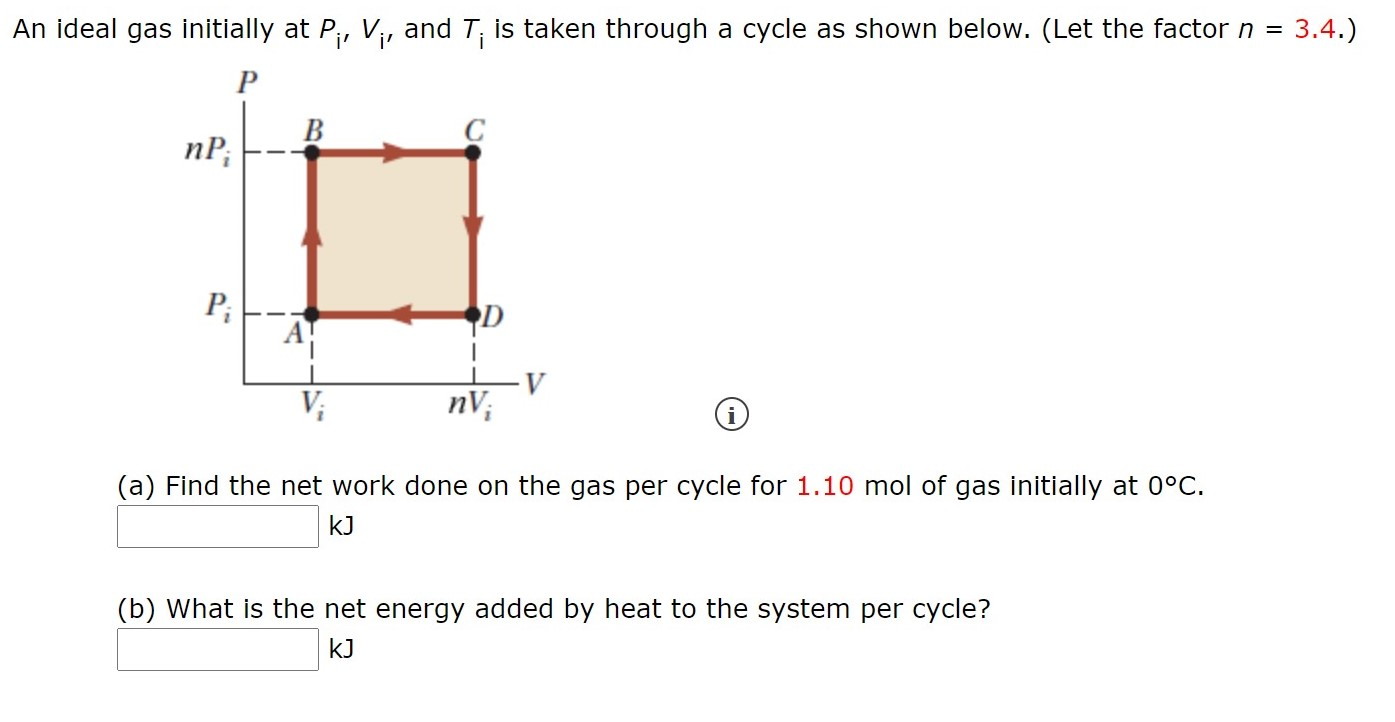
Solved An ideal gas initially at Pi, V;, and T; is taken

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
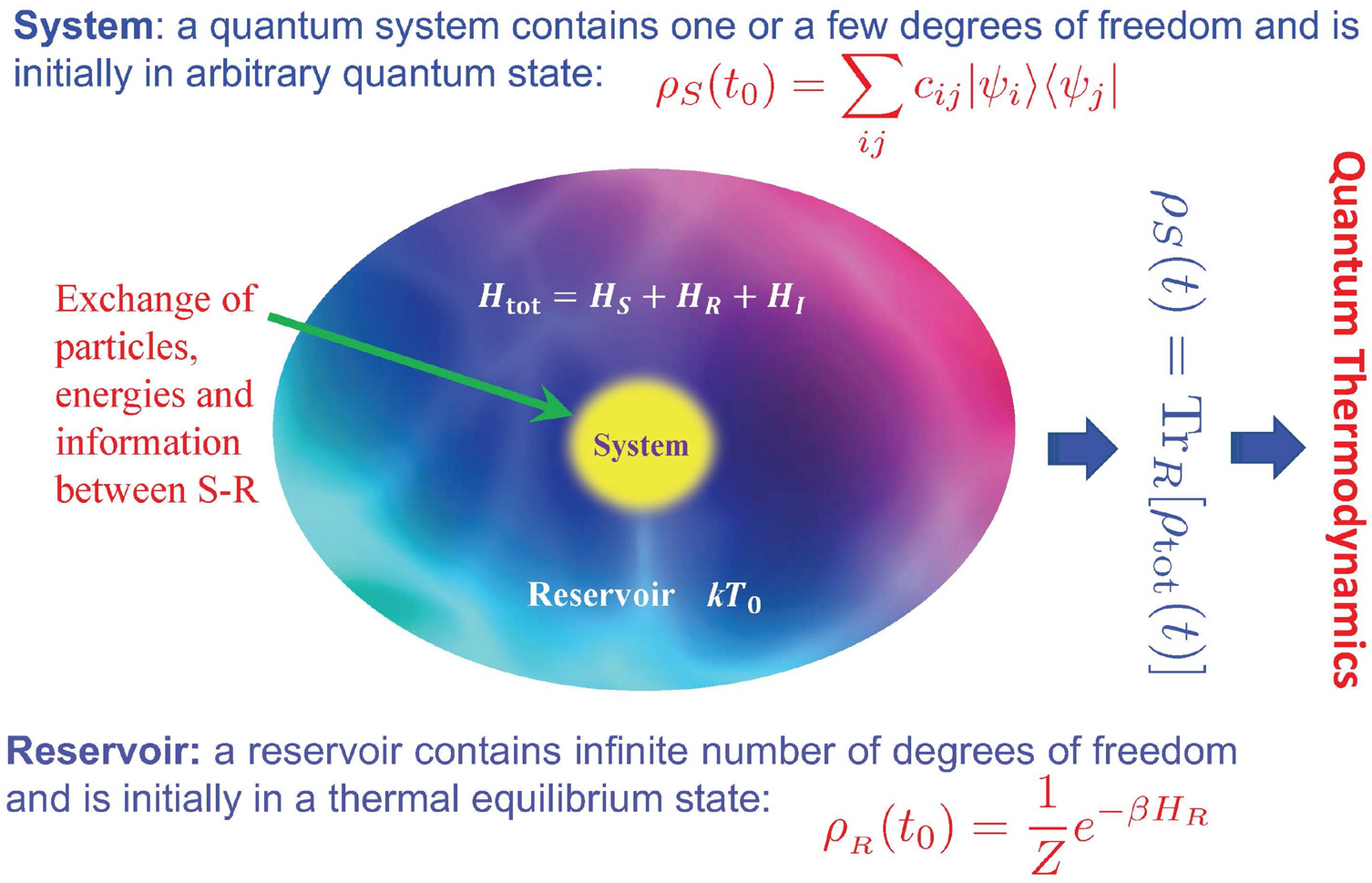
Quantum thermodynamics of single particle systems

1 mole of an ideal gas undergoes reversible isothermal expansion from an initial volume V_{1} to a final volume 10V_{1} and does 10 KJ of work. The initial pressure was 1times 10^{7}PaCalculate V_{1}
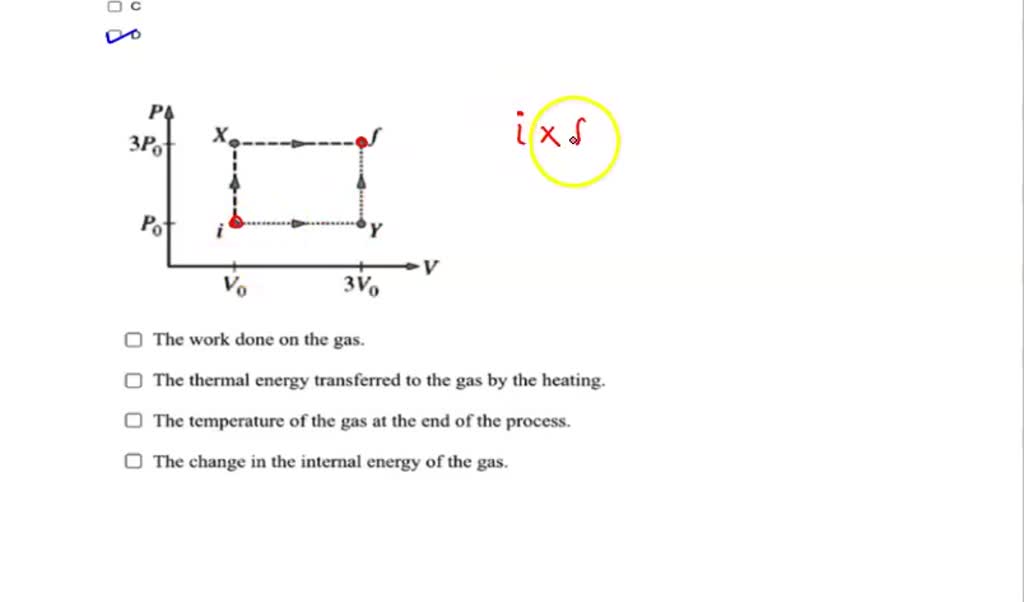
SOLVED: a. Identical samples of gas initially have pressure P, volume Vo, and temperature Tv. In some experiments, students take samples through each of the processes shown in the graph above. The
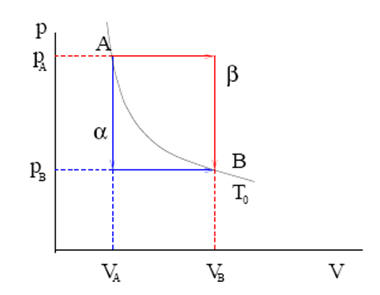
1st law
 Vanity Fair Womens Wireless Beyond Comfort Bra
Vanity Fair Womens Wireless Beyond Comfort Bra Travelers Collection Embroidered Jacket - Chico's
Travelers Collection Embroidered Jacket - Chico's Schaub Lumiere Collection 6 in. (152mm) Adjustable Clear Acrylic
Schaub Lumiere Collection 6 in. (152mm) Adjustable Clear Acrylic So Hot Right Now: Has Climate Change Created A New Literary Genre
So Hot Right Now: Has Climate Change Created A New Literary Genre Calca legging fitness academia levanta bumbum kaisan cintura alta diversas cores
Calca legging fitness academia levanta bumbum kaisan cintura alta diversas cores Visual Comfort Studio One Light Wall Sconce in Midnight Black and Burnished Brass
Visual Comfort Studio One Light Wall Sconce in Midnight Black and Burnished Brass