Solved The compression factor for a gas is 0.79 at 300 K and
5 (114) In stock

⏩SOLVED:Methane at 10 MPa and 300 K is heated at constant pressure…

Hydrogen storage - Wikipedia
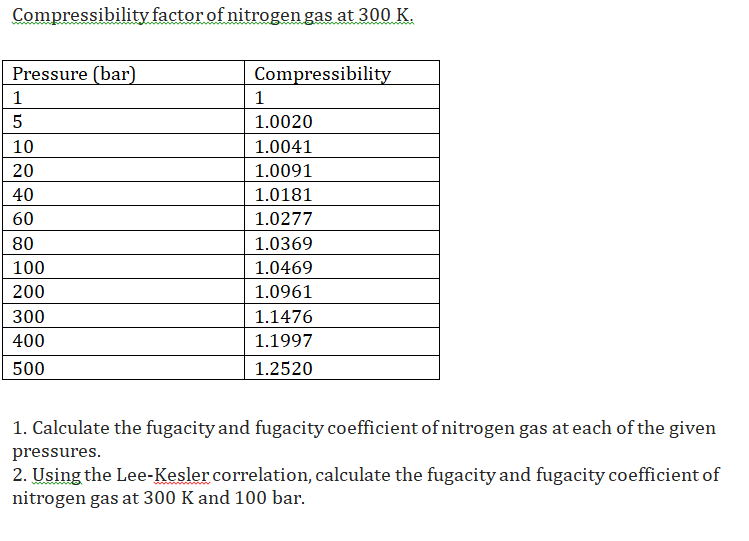
Solved Compressibility factor of nitrogen gas at 300 K.

Unit 8- BEHAVIOR OF GASES.ppt

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Physical Chemistry The Compression Factor (Z) [w/1 example]

Calculate the compressibility factor for a gas, if 1 mole of it occupy 0.821 litre at 300 K and 50 atm.A. 1.33B. 1.67С. 0.67D. 1
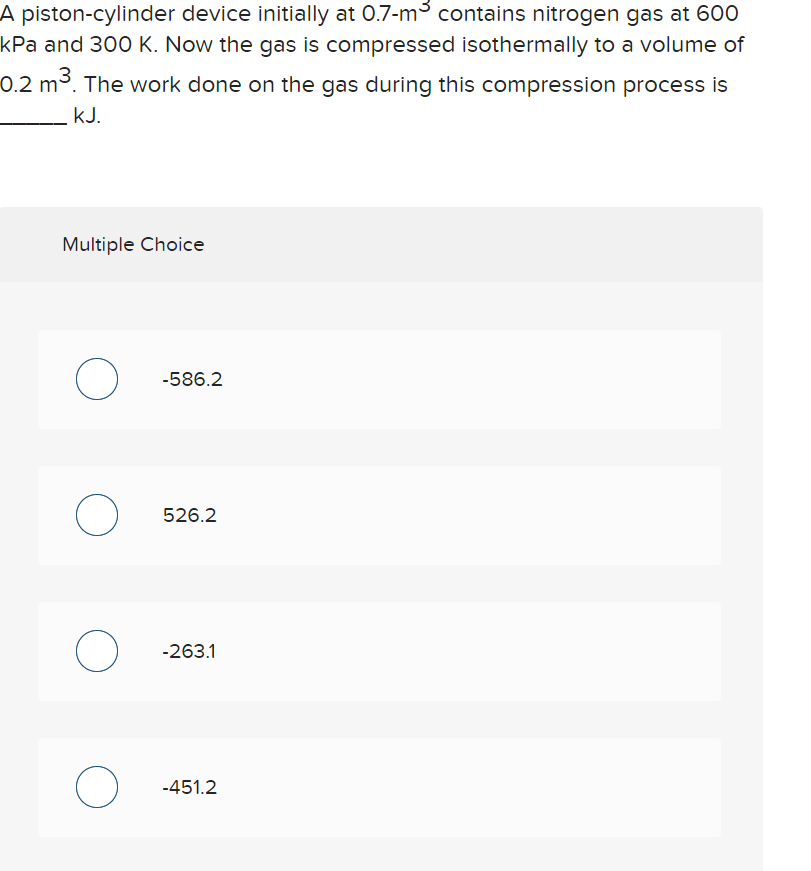
Solved A piston-cylinder device initially at 0.7-mºcontains
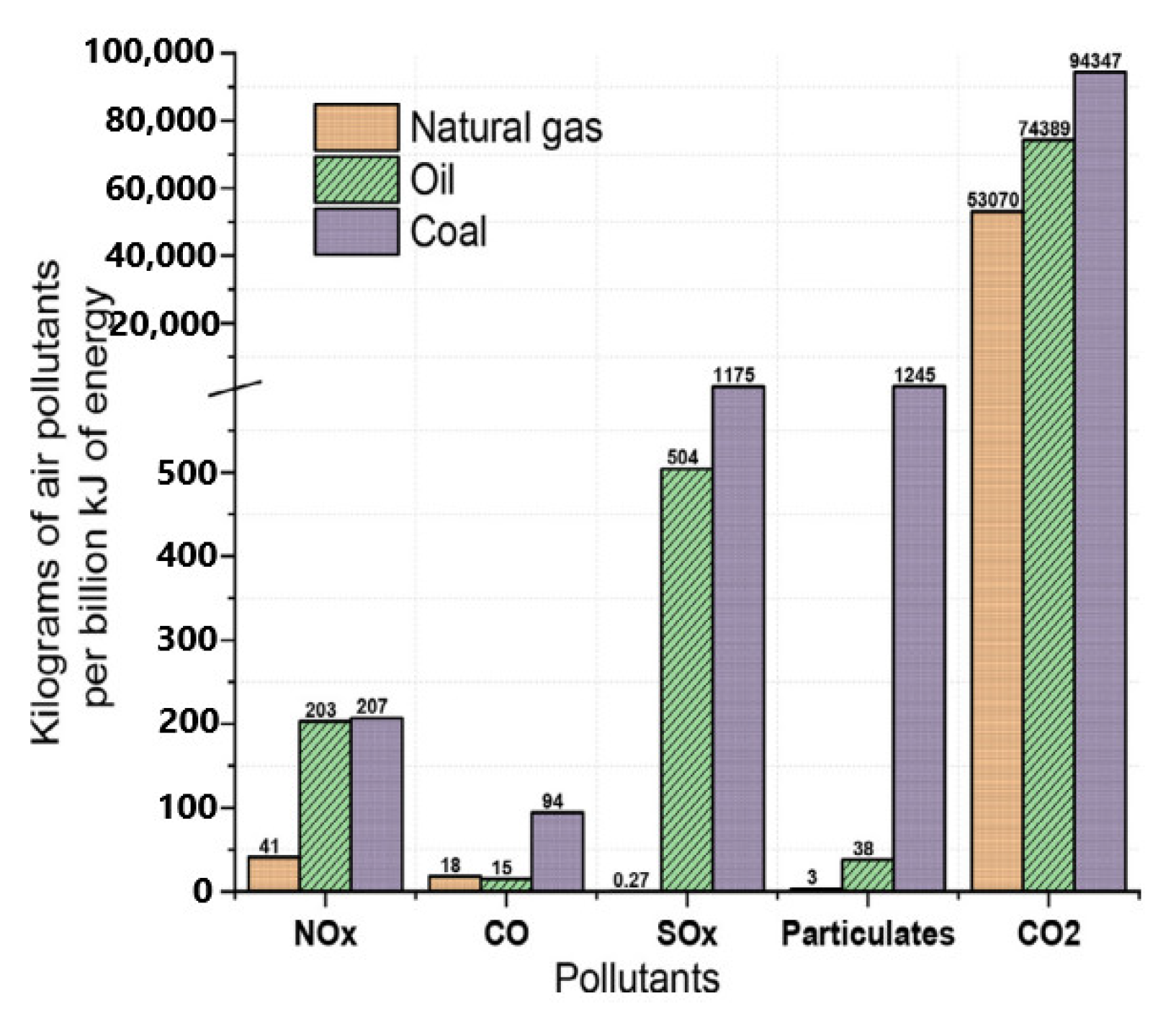
Energies, Free Full-Text
Solved 9 Compression factor Z Use the van-der-Waals equation
Solved The Berthelot equation of state is given by
At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{
 2-in-1 Hat Stand
2-in-1 Hat Stand Kaufe Große Winter-Lederleggings mit hoher Taille und hohem Bund
Kaufe Große Winter-Lederleggings mit hoher Taille und hohem Bund KAULIKE WOMEN'S BLACK & WHITE CAMI JUMPSUIT – Hawaii's Finest
KAULIKE WOMEN'S BLACK & WHITE CAMI JUMPSUIT – Hawaii's Finest The image of the skull of neon purple lamps with a bright glow on
The image of the skull of neon purple lamps with a bright glow on 1003 MagicLift Wireless Minimizer Bra 1003 - White – Purple Cactus
1003 MagicLift Wireless Minimizer Bra 1003 - White – Purple Cactus Janisramone Womens Shiny American Metallic Skinny Disco Leggings Wet Look Stretch Pants at Women's Clothing store
Janisramone Womens Shiny American Metallic Skinny Disco Leggings Wet Look Stretch Pants at Women's Clothing store