The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
4.9 (655) In stock
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

the vapour pressure of 2% aqueous solution of a non volatile substance X at 373 k is 755 torr . Calculate

16. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g of CS, (vapour pressure = 854 torr) is 848.9

Solutions (1-47) - Final, PDF, Solubility


fraction olligative Properties, Abnormality in Molar Mass) uids has a boi..

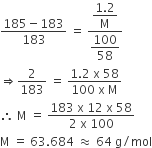
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o

Chapter-10 Solutions.pdf - Chemistry - Notes - Teachmint

Telugu] The vapour pressure of a solution having 2.0g of a solute X (
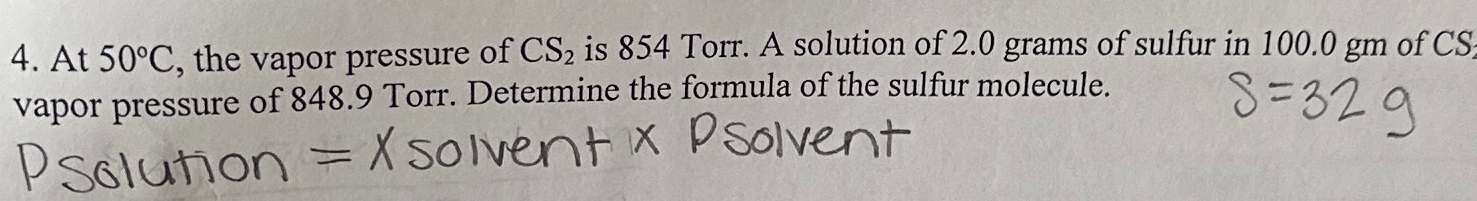
Solved At 50°C, the vapor pressure of CS2 is 854 Torr. A

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..
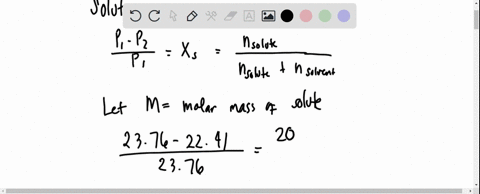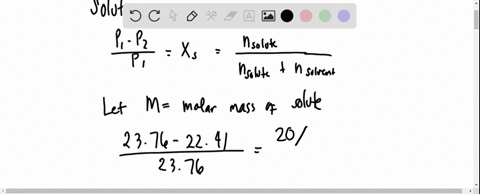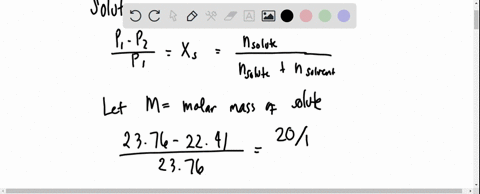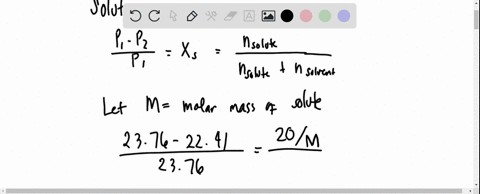
SOLVED: The vapour pressure of a solution having 2.0 g of solute X
Fralda Babysec Ultrasec Mega G 32 Unidades - Softys - Mobile
Babysec Ultrasec Galinha Pintadinha - Fralda, Tamanho G, 32 Unidades
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
Buy Amul Butter Cookies 32 g (Pack) Online at Best Prices in India
 Aqua-Dynamic Galvanized Black Iron Pipe Nipple, Male Pipe Thread, 3/4 x 24-in
Aqua-Dynamic Galvanized Black Iron Pipe Nipple, Male Pipe Thread, 3/4 x 24-in IMMI Boat Buckle F08893 G2 Retractable Boat Transom Tie Down
IMMI Boat Buckle F08893 G2 Retractable Boat Transom Tie Down Bra alteration before and after - making a pointy bra shape more
Bra alteration before and after - making a pointy bra shape more Atualização de Preços nas Opções de Assinatura, Tempo de jogo e
Atualização de Preços nas Opções de Assinatura, Tempo de jogo e DMC
DMC) Buy Kavanng Women's Alpine Nighty - with Mini Flower Design and Beautiful Round Neck Pattern, Premium Cotton Material Feel Comfortable Night Gown, Alpine Maxi (L, Maroon) Online at Best Prices in India
Buy Kavanng Women's Alpine Nighty - with Mini Flower Design and Beautiful Round Neck Pattern, Premium Cotton Material Feel Comfortable Night Gown, Alpine Maxi (L, Maroon) Online at Best Prices in India