SOLVED: For a gas at a given temperature, the compression factor
4.5 (709) In stock

VIDEO ANSWER: Hello students: let's look at the question: l n, that integrate integration and 0 z minus 1 bracket, close d p by p here. Minus 1 is equal to minus 8.50 into 10 to the power minus 3 p by p, not plus 3.50 into 10. To the power minus 9. P
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
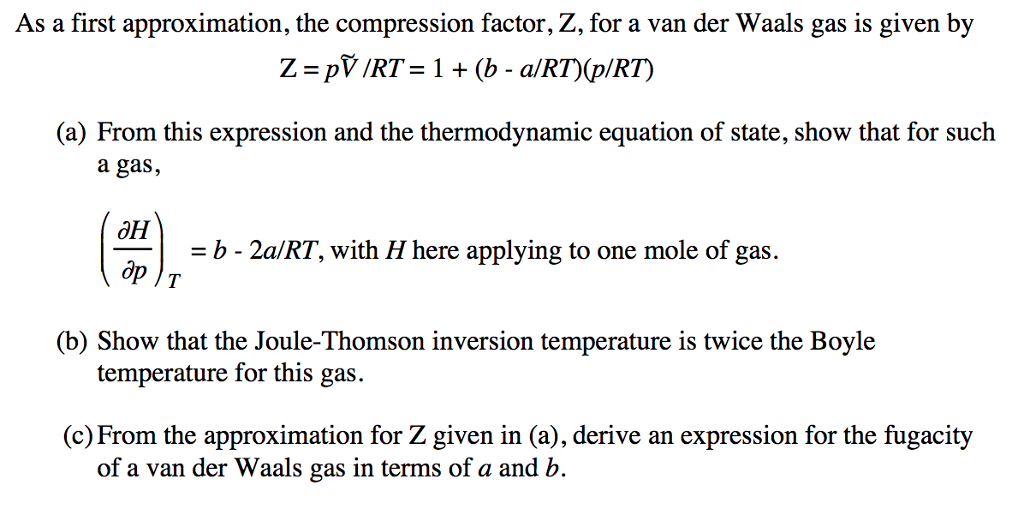
Solved As a first approximation, the compression factor, Z

Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes!
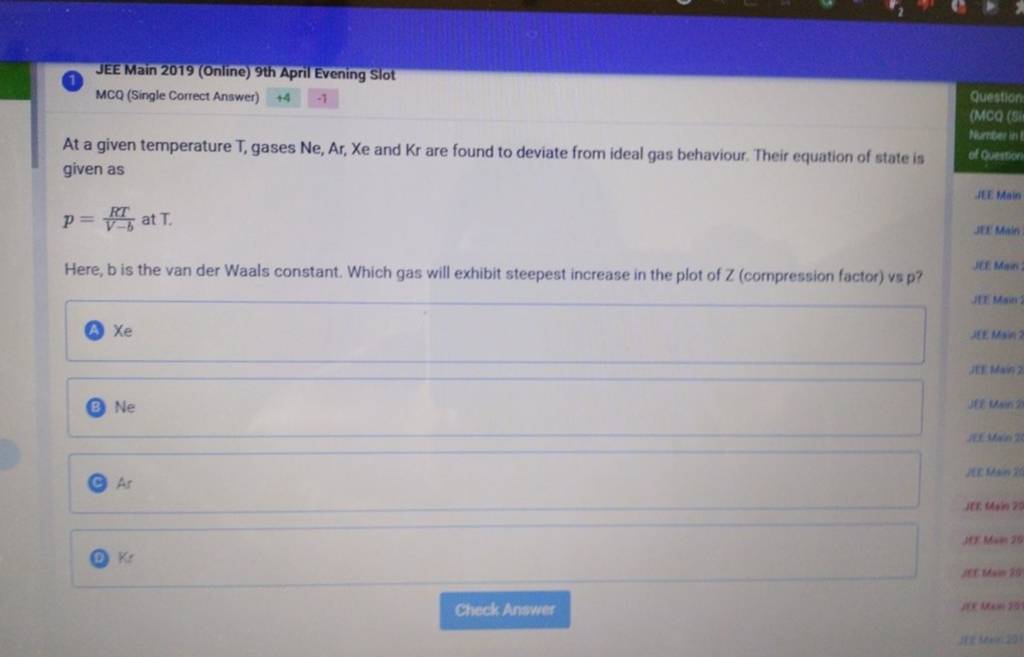
At a given temperature T, gases Ne,Ar, Xe and Kr are found to

6.3: Van der Waals and Other Gases - Physics LibreTexts

9.6 Non-Ideal Gas Behavior

Thermodynamics - 3-7 Ideal Gas Equation with compressibility
At Critical Temperature,pressure and volume . The compressibility

SOLVED: For a gas at a given temperature, the compression factor

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

The compression factor (compressibility factor) one mole of a van
Solved 9 Compression factor Z Use the van-der-Waals equation
Chapter 8 Real Gases. - ppt download
Compression Factor and Fugacity
Pick only the incorrect statement.for gas A, a=0,the
If `Z` is a compressibility factor, van der Waals' equation at low
 Under Armour Women's UA Surge 3 Running Shoes, UNBOXING
Under Armour Women's UA Surge 3 Running Shoes, UNBOXING LYSA, Love Your Size Always
LYSA, Love Your Size Always 1,200+ Black White And Grey Camouflage Pattern Stock Photos, Pictures & Royalty-Free Images - iStock
1,200+ Black White And Grey Camouflage Pattern Stock Photos, Pictures & Royalty-Free Images - iStock Cotton On Body Active The Recycled Mother Puffer Jacket 3.0 in Pink
Cotton On Body Active The Recycled Mother Puffer Jacket 3.0 in Pink Mudimba Woman Wearing A Bra Holding Her Baby, Village Of Combelo, Angola Stock Photo - Alamy
Mudimba Woman Wearing A Bra Holding Her Baby, Village Of Combelo, Angola Stock Photo - Alamy Gender Neutral Panties Green Cycling Shorts Underwear Teen Blue Underwear Disposable Maternity Knickers Black Ladies L : : Fashion
Gender Neutral Panties Green Cycling Shorts Underwear Teen Blue Underwear Disposable Maternity Knickers Black Ladies L : : Fashion