Establishing expiry date for clinical diagnostic reagents
4.9 (151) In stock

Product shelf life is an essential product performance requirement that, along with other design requirements, is used to determine the safety and efficacy of a clinical diagnostic
/tuv-rheinland-ivdr-visual-1-en.png)
In Vitro Diagnostic Medical Device Regulation (IVDR), IN

Clinical Chemistry Reagent Alkaline Phosphatase For Diagnostic

Chemicals and Reagents Management in Quality Control Laboratory
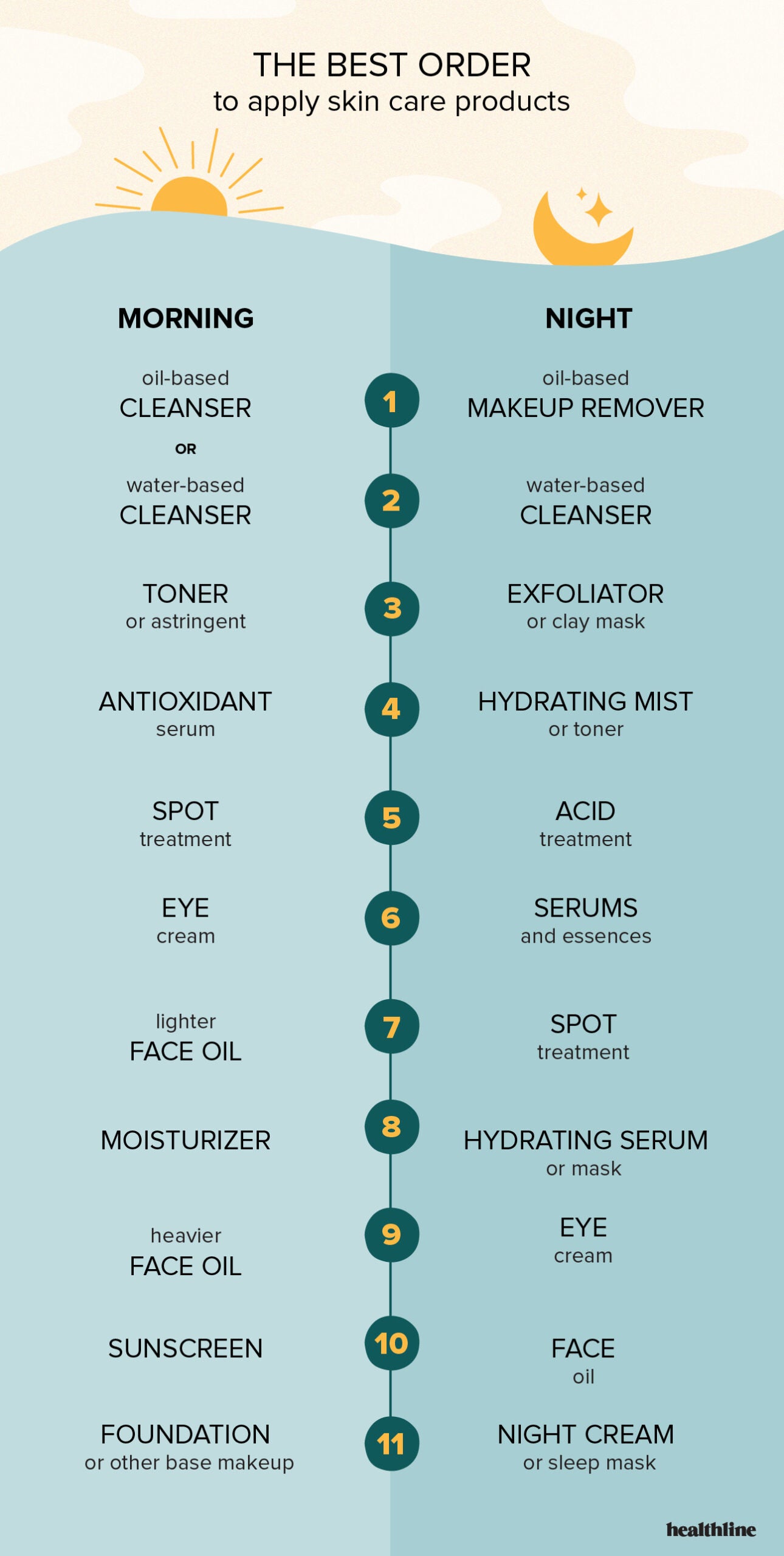
Skin Care Routine: What Is the Correct Order?

Alcohol urine test
Can You Still Use an Expired COVID-19 Test?

Establishing expiry date for clinical diagnostic reagents

Implementing a resource management program for accreditation

Scientific method - Wikipedia

Hemoglobin measurement methods
Food Expiration Date Guidelines Plus Easy-to-Read Chart
Ottawa urged to look into best-before date system to reduce grocery waste
BEEP - Expiry Date Tracking - Apps on Google Play
BEEP - Expiry Date Barcode Scanner
SS: Product Expiration Dates - Shelf life & best by date management for inventory
- El Closet De Mariele - LLEGARON 2 CAJAS 📦🇱🇷🇨🇴 -CAJA
 Hiphugger Underwear for Bladder Leaks
Hiphugger Underwear for Bladder Leaks Embrace Flared High Jeans
Embrace Flared High Jeans Lands' End Women's Plus Size Sport Knit High Rise Corduroy
Lands' End Women's Plus Size Sport Knit High Rise Corduroy Camellia Flowers Tattoo Sunflower Floral Butterfly Moon Rose Bird
Camellia Flowers Tattoo Sunflower Floral Butterfly Moon Rose Bird Buy Lipsy Black Sculpt Tummy Control Wear Your Own Bra Shapewear
Buy Lipsy Black Sculpt Tummy Control Wear Your Own Bra Shapewear
