SOLVED: Question: Using the equation for the compressibility factor, Z, calculate the value of Z for water at p = 35 MPa and 500°C (where the constant for steam is R =
4.6 (231) In stock


Thermodynamics - 3-7 Ideal Gas Equation with compressibility

Water vapor initially at 3.0 MPa and 300C (state 1) is contained

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor

Compressibility Factor - Thermodynamics I, EGN 3343
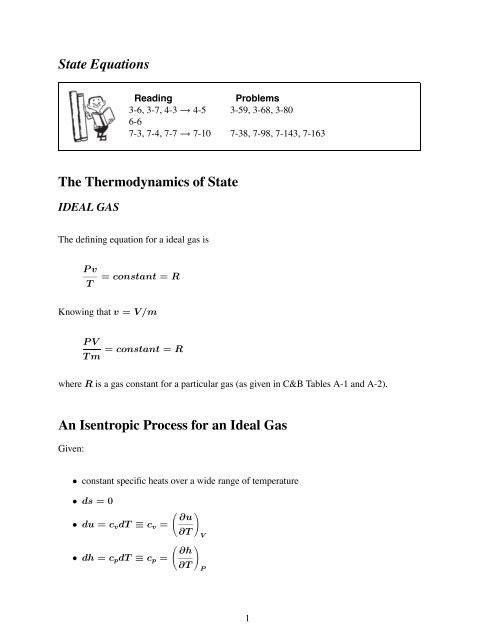
An Isentropic Process for an Ideal Gas

SOLVED: Using the equation for the compressibility factor, Z
3.3: Real gas and compressibility factor - Engineering LibreTexts

The compressibility factor for a real gas is expressed by, z =1+ BP / RT. The value of B at 500 K and 600 bar is 0.0169 L / mol. Find the

Thermodynamics: An Engineering Approach - 5th Edition - Part II by

Solutions manual for White Fluid Mechanics, Hector Novoa

P k nag solution by Shaikh Mohd Aslam - Issuu

Thermodynamics - 3-7 Ideal Gas Equation with compressibility

How to find Compressibility Factor Z
Summary of Equations used to evaluate compressibility factor, z
Non-Ideal Gas Behavior Chemistry: Atoms First
Compression Factor Calculator - Calculator Academy
PPT - The Ideal Gas PowerPoint Presentation, free download - ID:6789672
 3 Day Artistica Yoga Festival 15th-17th March 2024 - Bend it like Buddha Yoga
3 Day Artistica Yoga Festival 15th-17th March 2024 - Bend it like Buddha Yoga How To Play The Banjo Intro From Love Story By Taylor Swift - Beginner Guitar Lesson On Easy Songs
How To Play The Banjo Intro From Love Story By Taylor Swift - Beginner Guitar Lesson On Easy Songs Pans Yoga - Temu
Pans Yoga - Temu Jean Coutu Ordre de Montréal
Jean Coutu Ordre de Montréal Gubotare Womens Yoga Pants Petite Bootcut Yoga Pants with Pockets
Gubotare Womens Yoga Pants Petite Bootcut Yoga Pants with Pockets Women's Athletic Apparel, Activewear & More, Champion
Women's Athletic Apparel, Activewear & More, Champion