Compression of a gas due to external pressure and the
4.6 (633) In stock


The external pressure needed to compress an ideal gas from 22 dm to 8 dm' work done is of 4.545 KJ is a) 3.03 <10 Nm? b) 2.03 <10 Nm c) 1 10 Nm? a) -3.4 10' Nm

Waldo QUIROZ, Professor (Full), PhD Chemistry
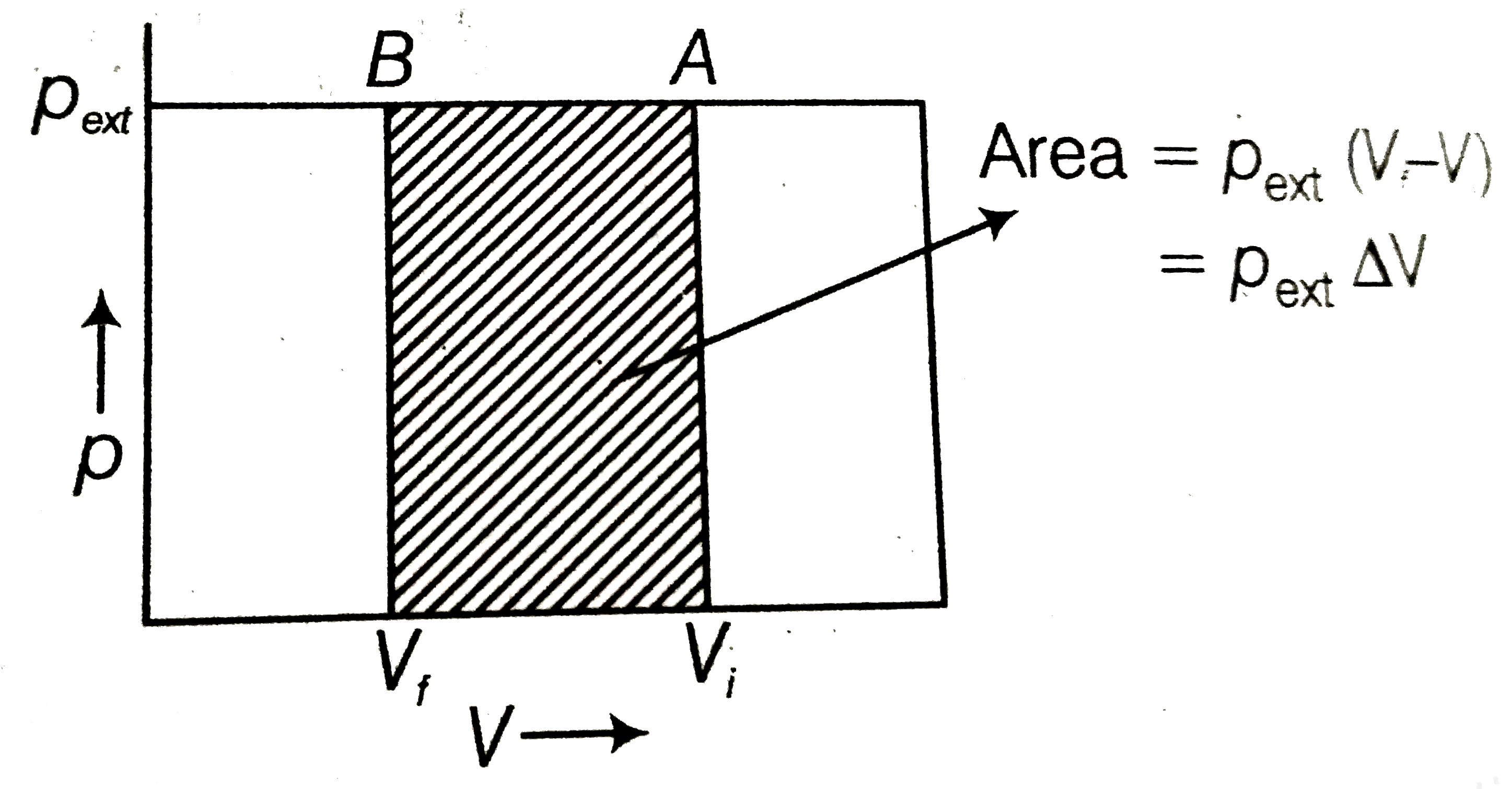
What will be the work done on an ideal gas enclosed in a cyliner, when

6) The work done (kJ) in the irreversible isothermal compression of 2.0 moles of an ideal gas from 1 bar to 100 bar 25°C constant external pressure of 500 bar is (A)

PDF) Natural laws and ontological reflections: the textual and

External pressure is greater than pressure of gas (system) So work is done 'on' the system So it - Chemistry - Thermodynamics - 10759473

Compression of a gas due to external pressure and the
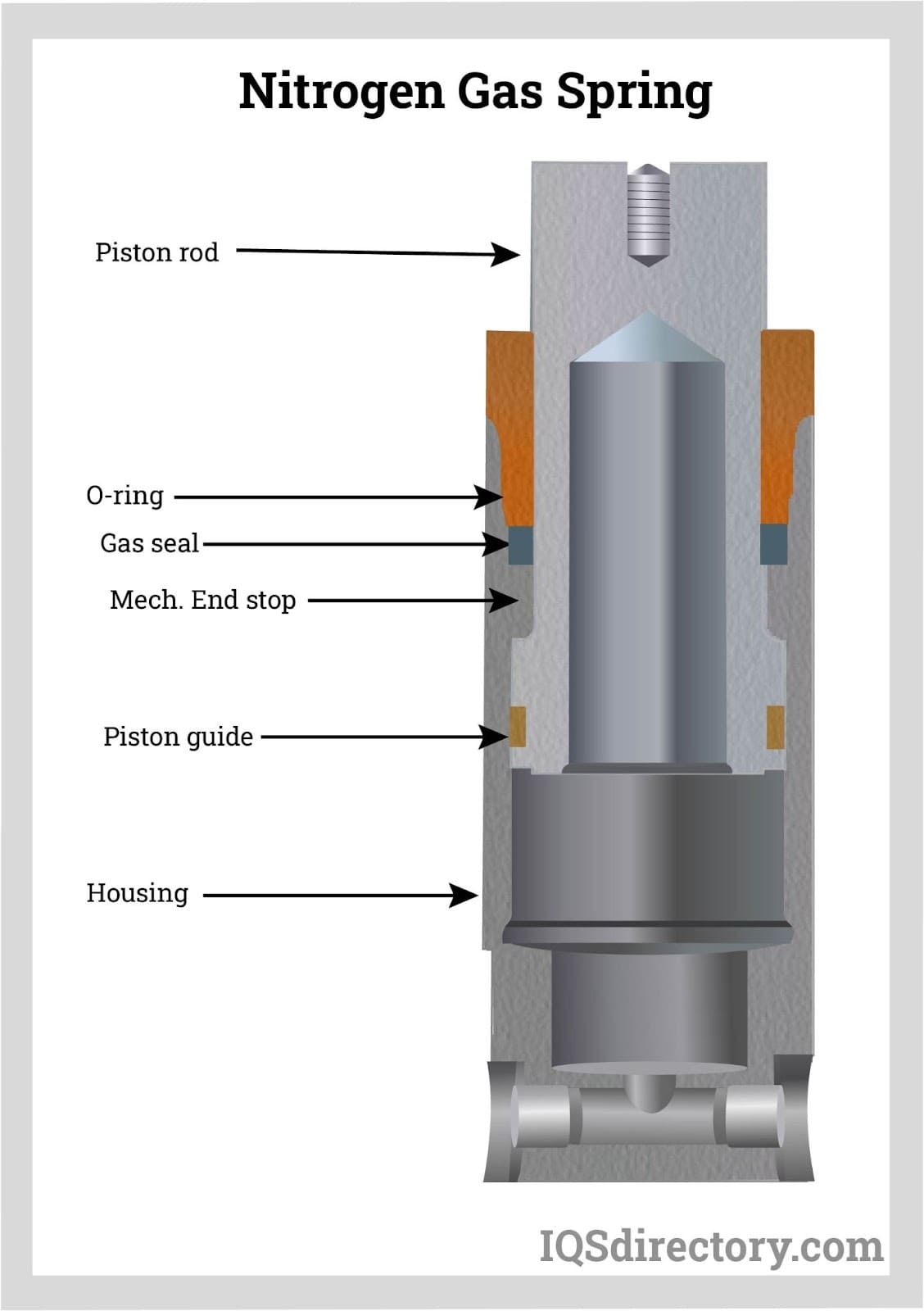
Gas Springs: Types, Design, Benefits, and Applications

1 (3) 248.5 K Pere R2- (4) 200 K La The work done in adiabatic compression of 2 mole of an ideal monoatomic gas by constant extemal pressure of 2 atm
Why do we use external pressure instead of internal pressure while calculating work done by a gas in an irreversible process? - Quora
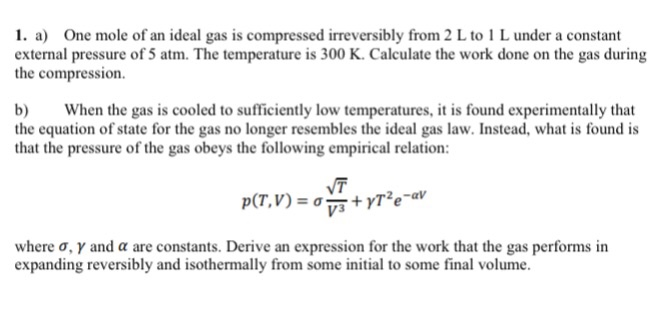
Solved 1. a) One mole of an ideal gas is compressed
Cranking pressure vs Compression Ratio - Motorcycle Engineering & Fabrication - ThumperTalk
ATS PRO DIFFERENTIAL PRESSURE TESTER WITH MASTER ORIFICE from Aircraft Tool Supply
AirWrap 4 Compression Bandage w/ Inflatable Bladder – Integrated
 Women's Fall Two-piece Outfit Alfred Dunner Black Elastic Waist Pants Size 16
Women's Fall Two-piece Outfit Alfred Dunner Black Elastic Waist Pants Size 16 ZHINIAN White Bra Top Underclothes Sports Bra Big Training Wireless Girls Student Cami Vest Cropped Bras Underwear for Teens Light Padded Bras
ZHINIAN White Bra Top Underclothes Sports Bra Big Training Wireless Girls Student Cami Vest Cropped Bras Underwear for Teens Light Padded Bras M.Rodini x Wrangler Adult Knickers Blue
M.Rodini x Wrangler Adult Knickers Blue How Bad Is It to Sit in Your Sweaty Underwear After Working Out?
How Bad Is It to Sit in Your Sweaty Underwear After Working Out? The Perfect Pair, Lovejoy Wiki
The Perfect Pair, Lovejoy Wiki Gift Items For Women
Gift Items For Women