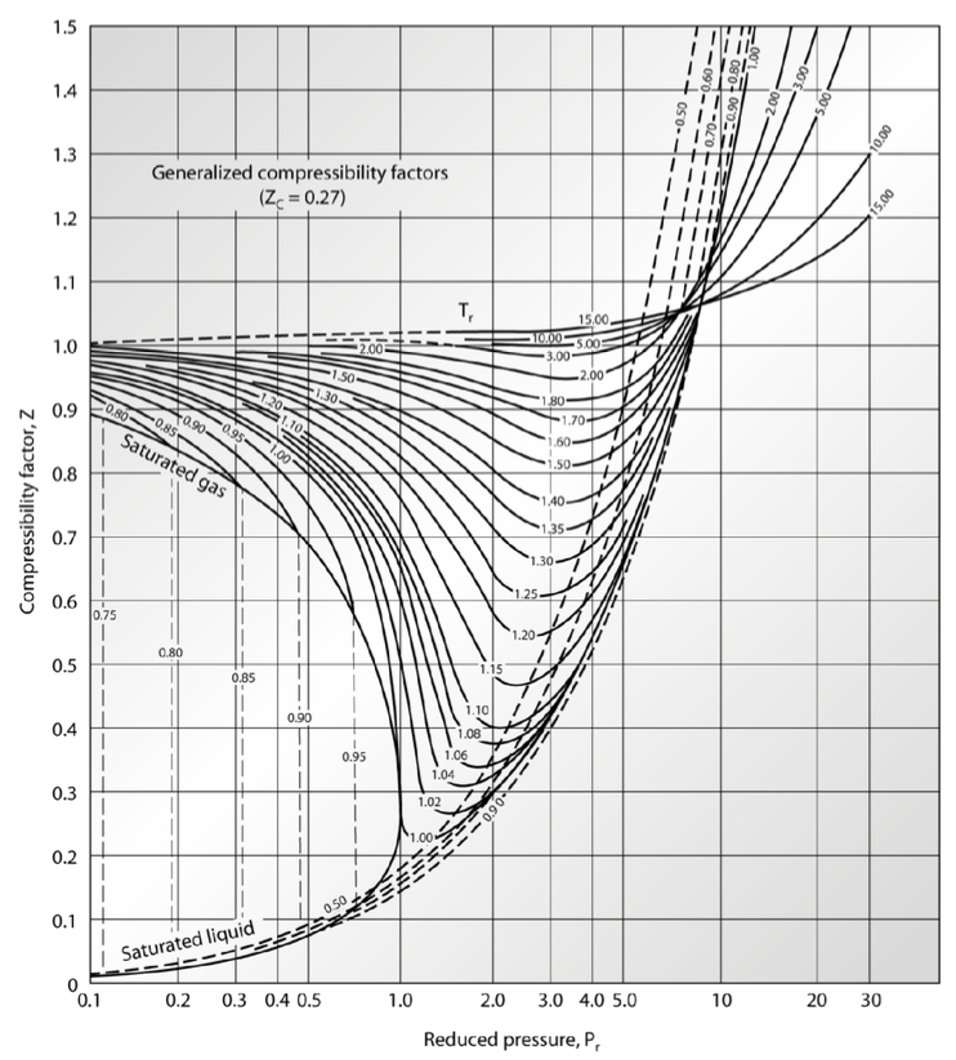
For a given gas, a graph is shown between compressibility factor
4.6 (559) In stock

By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.

Compressibility Factor Charts - Wolfram Demonstrations Project

Compressibility factor - Wikipedia

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?

Variation of both compressibility factor and viscosity of gas with

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following d
Qin Lab - thermal data
JEE Advanced Practice Test- 2 Free MCQ Practice Test with Solutions - JEE

e Compressibility factor (Z) for hydrogen WRT pressure and temperature

Thermodynamics Fundamentals

Left panel: Compressibility factor Z of the gas consisting from the

Compressibility factor Z is plotted against pressure p for four different gases A,B, C D. The correct order of critical temperature of the gases shown in the above graph is

Deviation Of Real Gas From Ideal Gas Behavior

Solved - Evaluate the compressibility factor of two
Compressibility Factor Calculator - File Exchange - MATLAB Central
Solved Using the chart, the compressibility factor (Z), for
Write the expression for the compressibility factor (Z) for one
 Jingle Bell Heist' Movie Casts Olivia Holt, Connor Swindells, Lucy Punch
Jingle Bell Heist' Movie Casts Olivia Holt, Connor Swindells, Lucy Punch Guely Ray Women Y Back Workout Tank Built in Bra Compression Long Yoga Camisole Top Shirt 1 Pack Blue Horizon XL Bust 37.5-39 Rib cage 31.5-33 in Kuwait
Guely Ray Women Y Back Workout Tank Built in Bra Compression Long Yoga Camisole Top Shirt 1 Pack Blue Horizon XL Bust 37.5-39 Rib cage 31.5-33 in Kuwait Arnott's Tim Tams vs McVitie's Penguin, by Corinna Bennett
Arnott's Tim Tams vs McVitie's Penguin, by Corinna Bennett Reversible Clothing for Women, Tops, Pants, Jackets
Reversible Clothing for Women, Tops, Pants, Jackets SLIM FIT SUIT PANTS - Tobacco
SLIM FIT SUIT PANTS - Tobacco Final Sale Plus Size 2pc Set Off The Shoulder Top & Leggings in
Final Sale Plus Size 2pc Set Off The Shoulder Top & Leggings in