Isolation, expansion and characterization of porcine urinary bladder smooth muscle cells for tissue engineering, Biological Procedures Online
4.7 (481) In stock
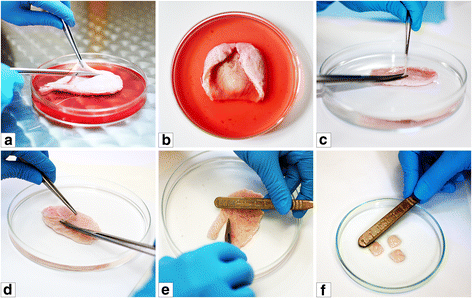
Background A key requirements for therapy utilizing the tissue engineering methodologies is use of techniques which have the capability to yield a high number of cells, from small tissue biopsy in a relatively short time. Up to date there was no optimal methods of isolation and expansion of urinary bladder smooth muscle cells (UB-SMCs). The aim of this study was to compare isolation and expansion techniques of UB-SMCs to select the most repeatable and efficient one. Method Five protocols of porcine UB- SMCs isolation including enzymatic and explant techniques and three expansion techniques were compared. Isolation effectiveness was evaluated using trypan blue assay. Cell phenotype was confirmed by immunofluorescence staining. Proliferation rate was analyzed using MTT and X- Celligence system. Cellular senescence was assessed measuring β-galactosidase activity. Results Enzymatic methods using collagenase with dispase (method I) or collagenase only (method III) allowed to isolate much larger number of cells than the methods using trypsin with collagenase (method II) and collagenase after digestion with trypsin (method IV). The success rate of establishment of primary culture was the highest when the isolated cells were cultured in the Smooth muscle Growth Medium-2 (SmGM-2). Expression of the smooth muscle markers- alpha smooth muscle actin and smoothelin was the highest for cells isolated by enzymatic method I and cultured in SmGM-2. There was no significant signs of cell senescence until the 8th passage. Conclusion The most efficient method of establishment of porcine UB-SMCs culture is enzymatic digestion of urinary bladder tissue with collagenase and dispase and culture of isolated cells in SmGM-2. This method was up to 10 times more efficient than other methods used for isolation and culture of UB-SMCs. This is an easy and consistent method for obtaining high numbers of urinary bladder smooth muscle cells.

Porous gelatin microspheres implanted with adipose mesenchymal stromal cells promote angiogenesis via protein kinase B/endothelial nitric oxide synthase signaling pathway in bladder reconstruction - Cytotherapy

Bioengineering, Free Full-Text

PDF) Isolation, expansion and characterization of porcine urinary bladder smooth muscle cells for tissue engineering
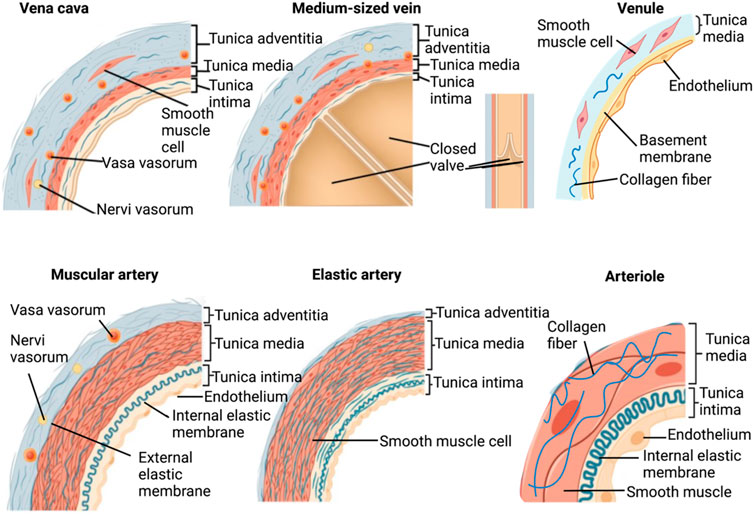
Frontiers Decellularized blood vessel development: Current state-of-the-art and future directions

Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract

Isolation, expansion and characterization of porcine urinary bladder smooth muscle cells for tissue engineering, Biological Procedures Online
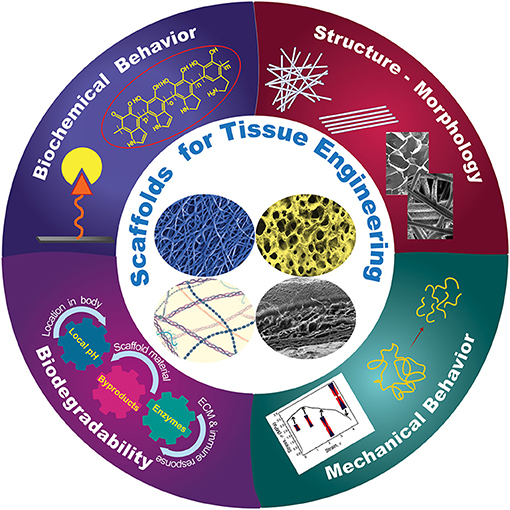
Frontiers Design Challenges in Polymeric Scaffolds for Tissue Engineering

IJMS, Free Full-Text

Engineering of the Bladder and Urethra

Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder

Applied Sciences, Free Full-Text

Urinary bladder and urethral tissue engineering, and 3D bioprinting approaches for urological reconstruction
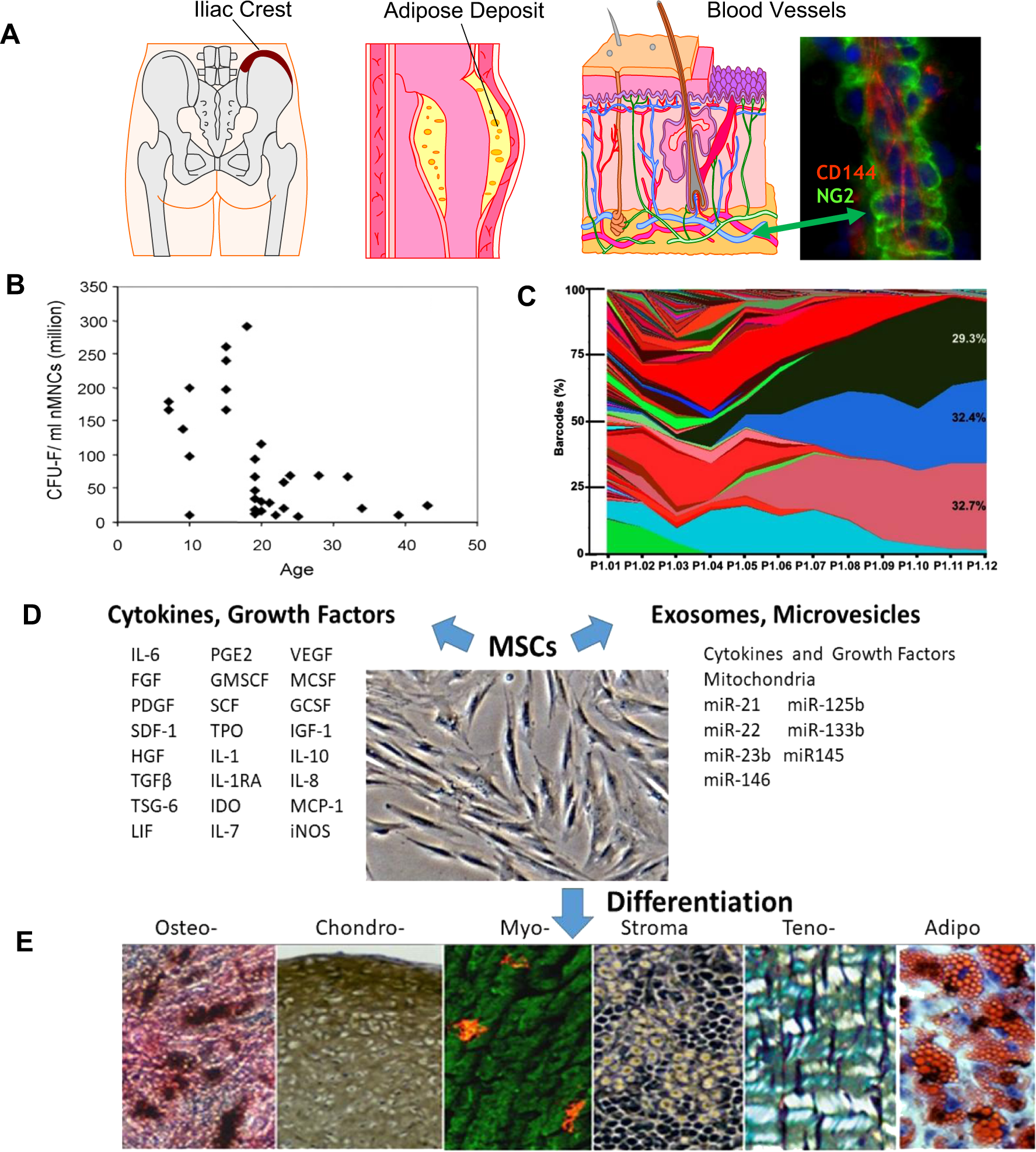
Mesenchymal stem cell perspective: cell biology to clinical progress

Urinary bladder and urethral tissue engineering, and 3D bioprinting approaches for urological reconstruction
 Members Mark Everyday High Rise Ankle Leggings Sz Small NWT Gray
Members Mark Everyday High Rise Ankle Leggings Sz Small NWT Gray Pull-Ups Learning Designs Training Pants for Boys, 3T-4T (Packaging May Vary) : : Baby
Pull-Ups Learning Designs Training Pants for Boys, 3T-4T (Packaging May Vary) : : Baby/product/15/7311331/1.jpg?2193) Shop Fashion Seamless Body Sculpting Shaper Womens Sleeeless Tummy Control Bodysuit Shapewear Crew Neck Racerback Tank Tops Snap Online
Shop Fashion Seamless Body Sculpting Shaper Womens Sleeeless Tummy Control Bodysuit Shapewear Crew Neck Racerback Tank Tops Snap Online 315,598 Zodiac Signs Vector Images, Stock Photos, 3D objects
315,598 Zodiac Signs Vector Images, Stock Photos, 3D objects Autumn Winter Warmer Corset Chest Wrap Soft Cotton Yarn Thin Women Knitted Underwear Shapewear Tube Top High Elasticity Seamless - AliExpress
Autumn Winter Warmer Corset Chest Wrap Soft Cotton Yarn Thin Women Knitted Underwear Shapewear Tube Top High Elasticity Seamless - AliExpress Victoria's Secret
Victoria's Secret