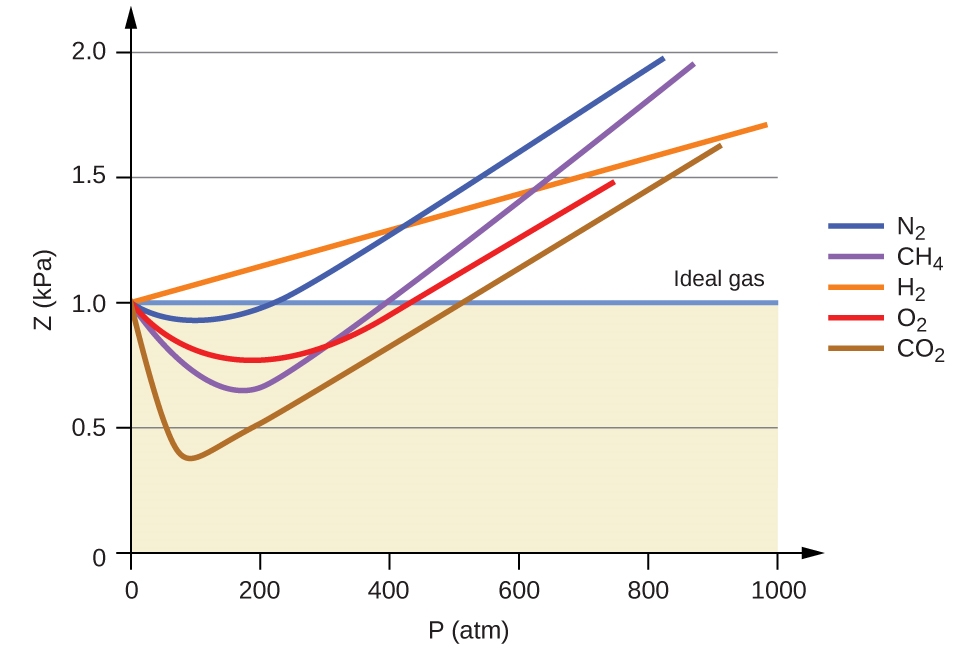
For H(2) gas, the compressibility factor,Z = PV //n RT is
4.7 (628) In stock

For H(2) gas, the compressibility factor,Z = PV //n RT is
2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Solved RT B 2. The compressiblity factor for a gas is

COMPRESSIBILITY FACTOR

Compressibility factor Z - Gaseous State
The given graph represent the variation of z compressibility factor z=pv/nRT versis p fpr three real gases A,B,C identify only incorrect statement

Deviation of Real Gases from Ideal Gas Behaviour - Chemistry for ACT PDF Download

6.3: Van der Waals and Other Gases - Physics LibreTexts
A: Compressibility factor for hydrogen varies with pressure, but always shows positive slope at0C. R: At low P, repulsive forces dominate in hydrogen gas at 0^° C. is R false)

Non-Ideal Gas Behavior Chemistry: Atoms First
Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1
Compressibility Factor Calculator - Community
3.3.3: Natural Gas Properties PNG 301: Introduction to Petroleum and Natural Gas Engineering
Thermodynamics - 3-7 Ideal Gas Equation with compressibility
 Promoção Red Paddle Co – 12'6 X 30” SPORT 2022 MSL
Promoção Red Paddle Co – 12'6 X 30” SPORT 2022 MSL Leggings Lululemon Multicolour size 4 US in Spandex - 40949027
Leggings Lululemon Multicolour size 4 US in Spandex - 40949027 The Dairy Fairy - Arden Nursing Bra
The Dairy Fairy - Arden Nursing Bra 90s PARISIAN PADDED BLUE ROSE DESIGNER BRA - (32A/30B/34AA)
90s PARISIAN PADDED BLUE ROSE DESIGNER BRA - (32A/30B/34AA) Dallas Cowboys Official Site of the Dallas Cowboys
Dallas Cowboys Official Site of the Dallas Cowboys Types of Figure Skating Tights - Houston Skate & Sports Orthotics
Types of Figure Skating Tights - Houston Skate & Sports Orthotics