Derive an expression for the compression factor of a gas tha
4.7 (416) In stock
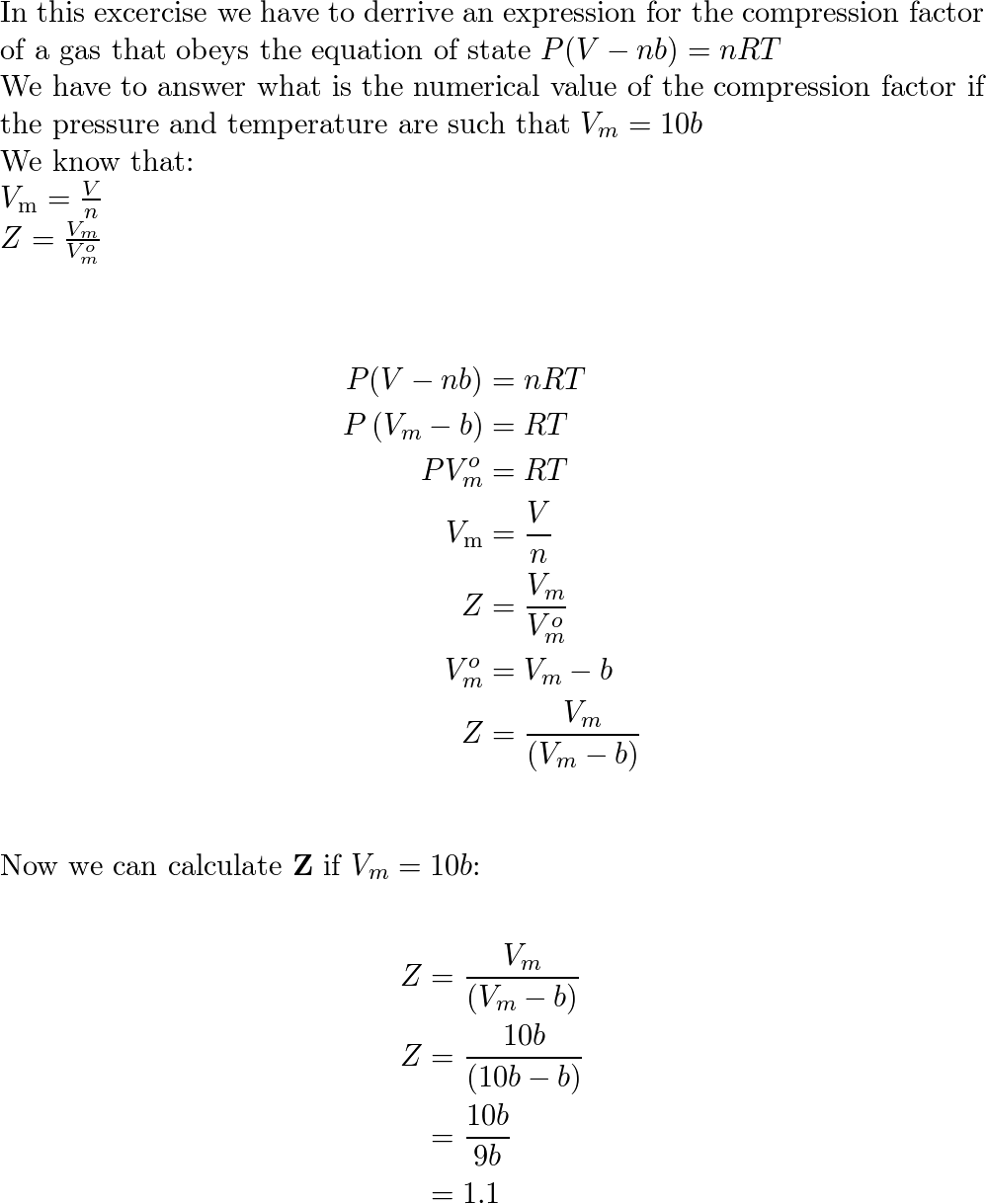

Methane Gas Volume Expansion Ratios and Ideal Gas Deviation Factors for the Deep-Water Bering Sea Basins: Peng-Robinson Equation of State

Degrees Conferred by Major ( ) Source: National Center for Education Statistics (NCES) Business 366,815 Social Sciences 178,543 Psychology 108, ppt download
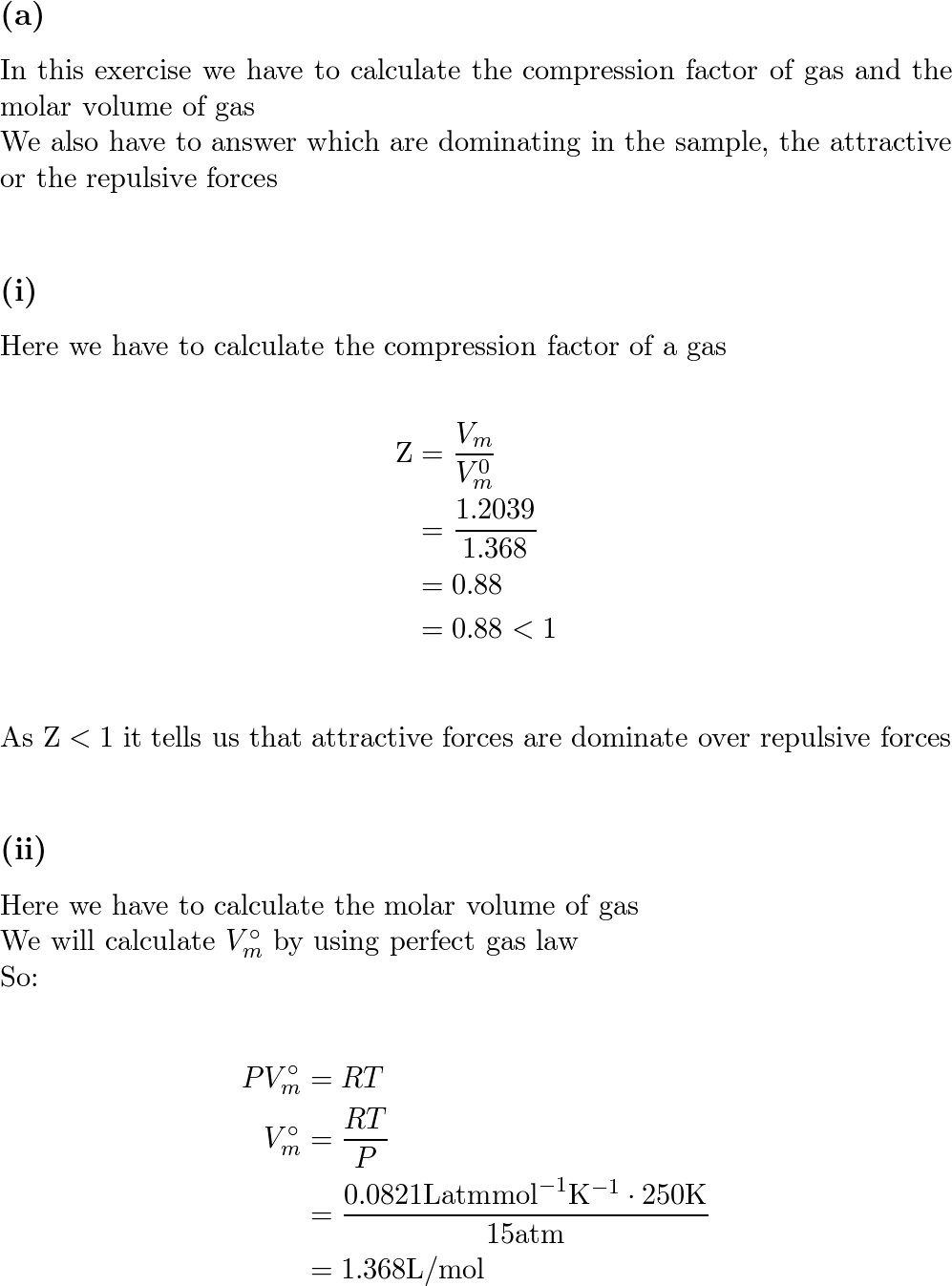
a) A gas at 250 K and 15 atm has a molar volume 12 per cent

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
Van der waals equation: Derivation, Explanation
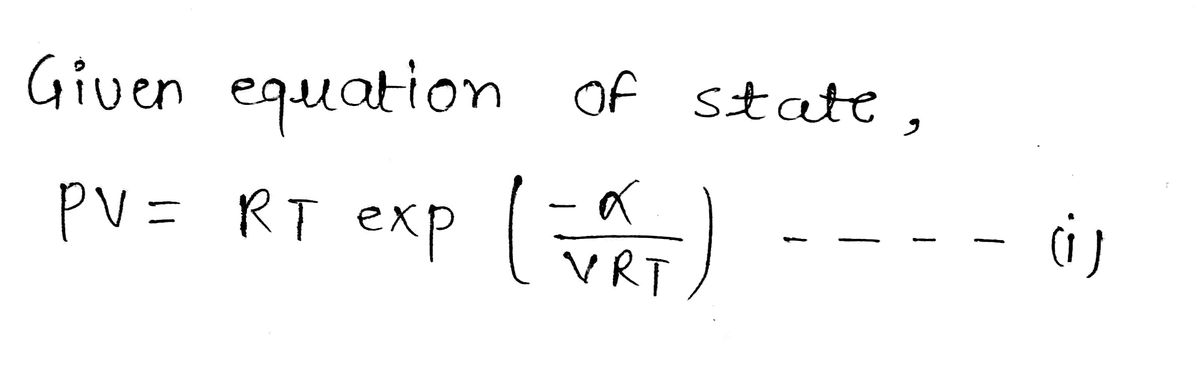
Answered: α = alpha A possible equation of state…
Solved] Consider a long cylindrical pipe of inner radius R1 and outer

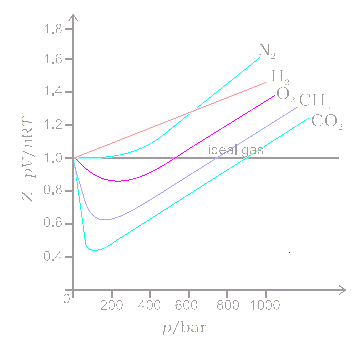
3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
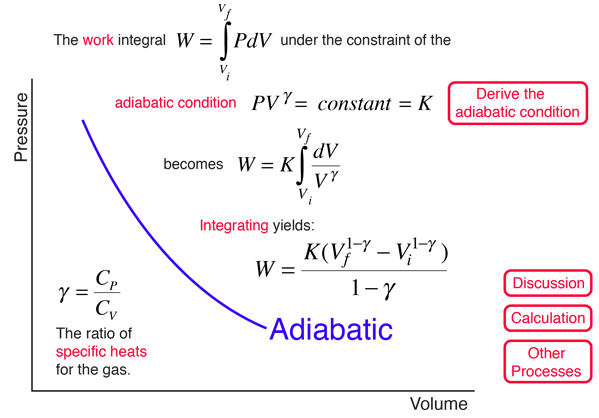
Adiabatic Processes
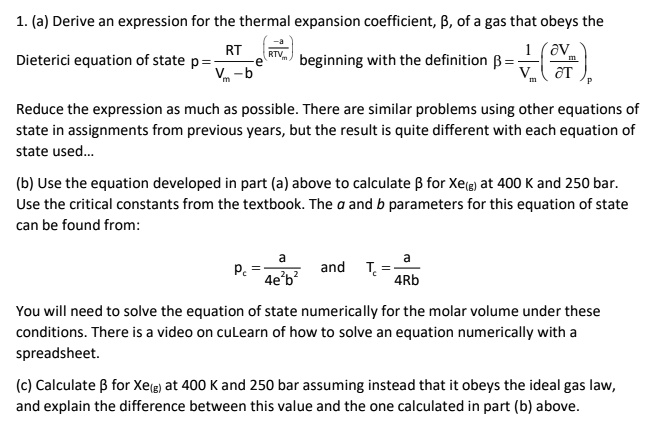
SOLVED: (a) Derive an expression for the thermal expansion coefficient of a gas that obeys the Dieterici equation of state, beginning with the definition α = (1/V)(∂V/∂T). Reduce the expression as much
Solved As a first approximation, the compression factor, Z
Find the isothermal compressibility `x` of a Van der Walls gas as a function of volume
If `Z` is a compressibility factor, van der Waals' equation at low
 Resultado da Super Sete 521 hoje (18/03/24); prêmio de R$ 2 milhões
Resultado da Super Sete 521 hoje (18/03/24); prêmio de R$ 2 milhões Woman Praying God, Religion,Believe,Latina ,Strong,African American, Black Woman, Classy, Glamour, Nubian, Princess, Queen, Diva | Art Print
Woman Praying God, Religion,Believe,Latina ,Strong,African American, Black Woman, Classy, Glamour, Nubian, Princess, Queen, Diva | Art Print MPG Sport Mondetta Performance Gear Travel Dress Purple : : Clothing, Shoes & Accessories
MPG Sport Mondetta Performance Gear Travel Dress Purple : : Clothing, Shoes & Accessories Travel Pants by Cordelia Street - Navy, Women's Basics
Travel Pants by Cordelia Street - Navy, Women's Basics Sexy Seamless Butt Lifting Leggings Fitness Sets High Waist Hip Raising Pants with Sports Bra Yoga Set Women - China Yoga Set and Yoga Sets price
Sexy Seamless Butt Lifting Leggings Fitness Sets High Waist Hip Raising Pants with Sports Bra Yoga Set Women - China Yoga Set and Yoga Sets price Which lululemon Scuba Oversized Zip is Right for YOU!? Comparing the 1/2 Zip and Full Zip / Fall2021
Which lululemon Scuba Oversized Zip is Right for YOU!? Comparing the 1/2 Zip and Full Zip / Fall2021