In the following compressibility factor (Z) vs. pressure graph 300
4.5 (433) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

If the slope of 'Z' (compressibility factor) vs. 'p' curve is constant `(slope=(pi)/(492.6)atm^
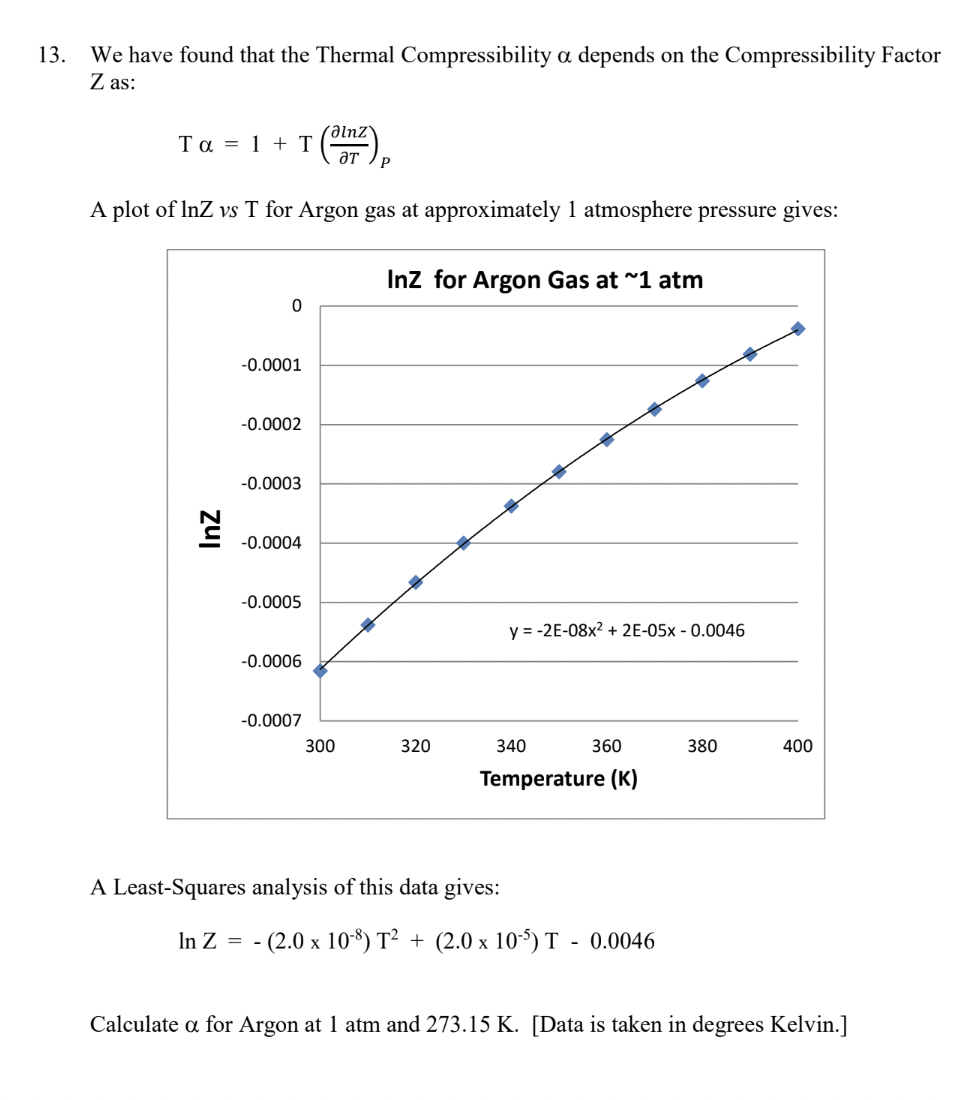
Solved 13. We have found that the Thermal Compressibility α

Compressibility factor Z - Gaseous State

physical chemistry - Compressibility Factor Graph - Which gas attains a deeper minimum? - Chemistry Stack Exchange

Energies, Free Full-Text

Compressibility factor of water vapor along its saturation curve. Error
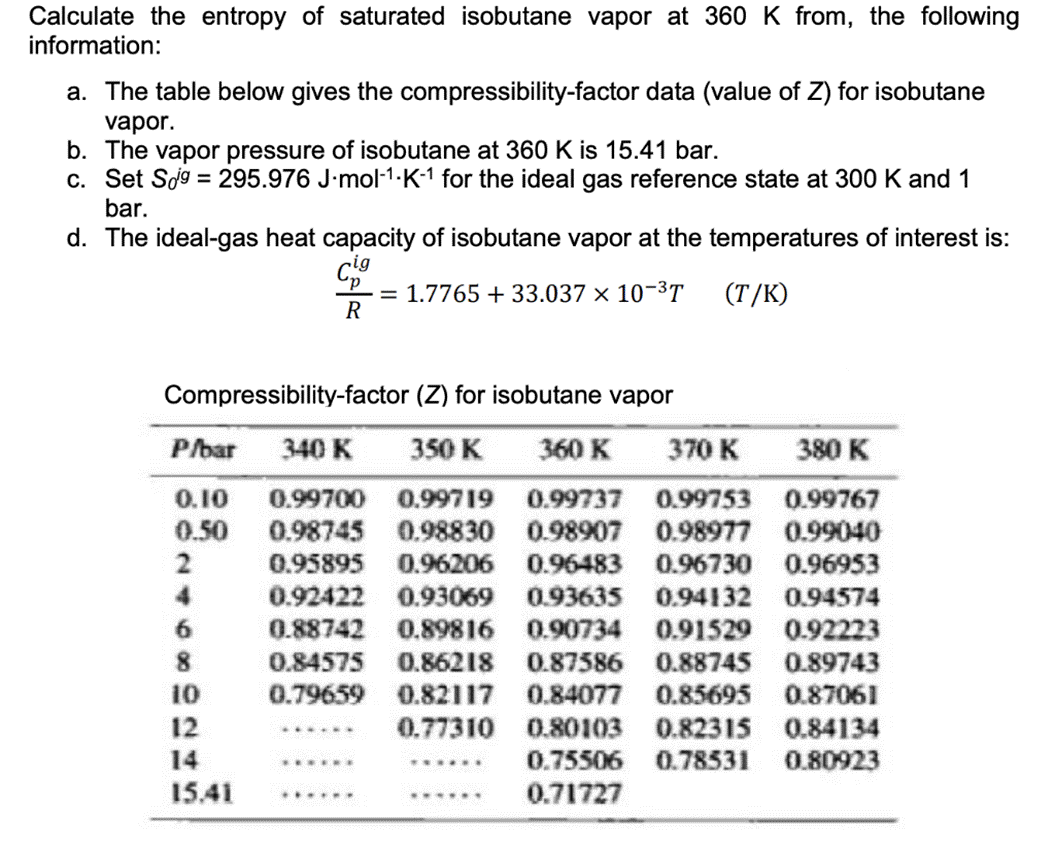
Solved) - Calculate the entropy of saturated isobutane vapor at 360 K from, (1 Answer)

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.

Compressibility Chart - an overview

The graph of compressibility factor Z vs P for one mole of a real gas is shown in following diagram. The graph is plotted at a constant temperature 273 K. If the
Compressibility factor (Z) is plotted against pressure at different te
Answer in Molecular Physics Thermodynamics for Neilmar #278440
Two extensions of the compressibility factor Z correlation (sub-critical pressure region)
thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange
- 1 PCS Hight Quality TR-414 Tyre Valve Germany Black Tubeless
 Unionbeauty Red Body Wave Braiding Hair 24 Inch 9 Packs Pre-Feathered Braiding Hair for Goddess Boho Box Braids Synthetic Crochet Braids Hair Crochet
Unionbeauty Red Body Wave Braiding Hair 24 Inch 9 Packs Pre-Feathered Braiding Hair for Goddess Boho Box Braids Synthetic Crochet Braids Hair Crochet Weight Training, Smith Machine
Weight Training, Smith Machine New Skechers Women's Shape Ups Metabolize Fitness Work Out Sneaker Size 10, $150
New Skechers Women's Shape Ups Metabolize Fitness Work Out Sneaker Size 10, $150 New Design 36 Size Bra Underwear - China Bra Set and Adjustable Sports Bra price
New Design 36 Size Bra Underwear - China Bra Set and Adjustable Sports Bra price Travel Accessories Eye Mask For Sleeping Travel Sleeping Eye Mask Deep Sleep Relaxing Soft Elasticated Sleeping Rest Aid Headache Puffines 1Pc - Black
Travel Accessories Eye Mask For Sleeping Travel Sleeping Eye Mask Deep Sleep Relaxing Soft Elasticated Sleeping Rest Aid Headache Puffines 1Pc - Black
