Consider the graph between compressibility factor Z and pressure P
4.6 (449) In stock

Z1 means force of attraction dominating ie a is considerable b can be negligible at low temperature and low pressure Lower is the value of Z easier is the process of liquification
The compressibility factor is actually a factor that corrects the actual value of the gas versus the ideal gas. Let us learn and understand this concept.
Watch this video to understand the behaviour of real gases with the help of the compressibility factor. This is an important topic for JEE main.
What is the compressibility factor, and how does it vary with an increase in temperature and pressure? Watch this video to get the answer. This is an importa
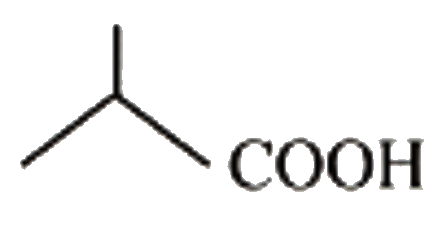
How many of the following acids will show higher reactivity towards es
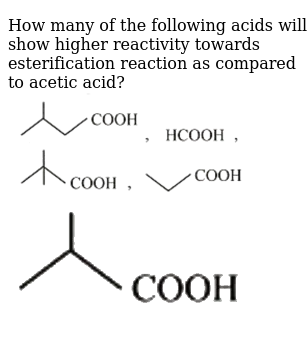
How many of the following acids will show higher reactivity towards es
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
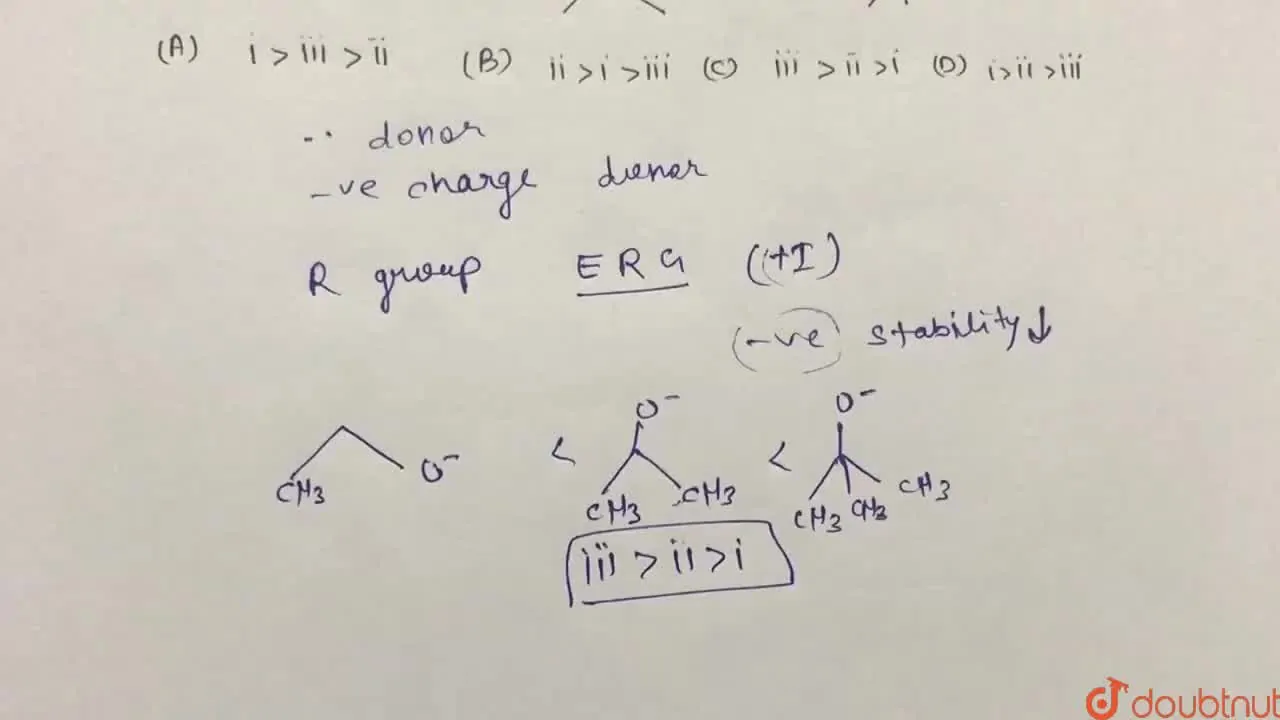
Determine the order of basic stregth of the given molecules

Gas Compressibility - an overview
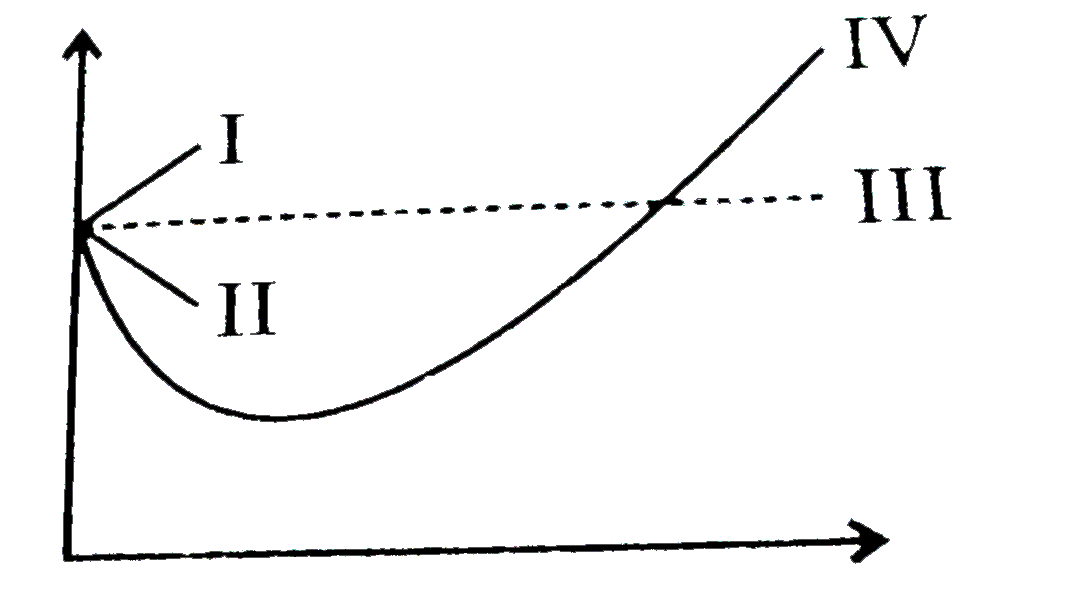
Telugu] The variation of compressibility factor (Z) with pressure (p
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!
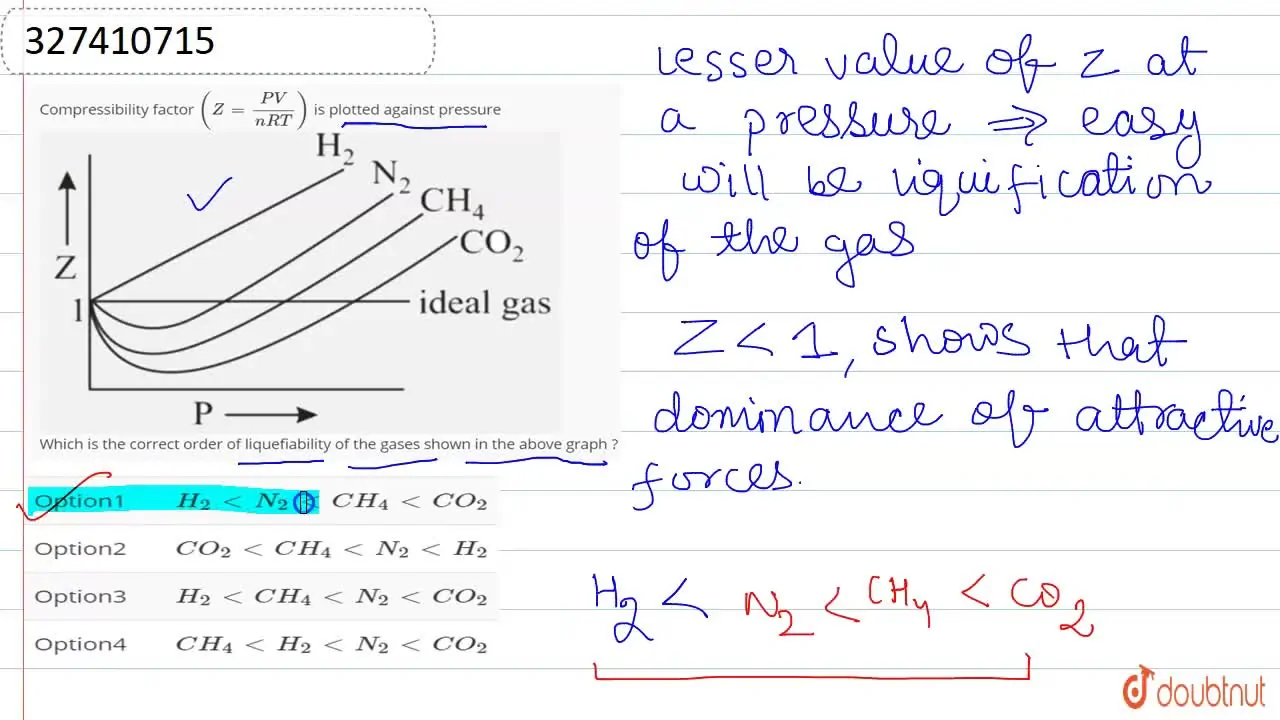
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
Physical Chemistry The Compression Factor (Z) [w/1 example]
Compressibility factor (z): real gases deviate from ideal behav-Turito
Answered: (a)Using the compressibility chart,…
Real Gases vs Ideal Gases & the Compressibility Factor
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created
 NEW PLAYTEX 18 HOUR SMOOTH APPEARANCE BLACK BRA 38B 42B - STYLE 4395
NEW PLAYTEX 18 HOUR SMOOTH APPEARANCE BLACK BRA 38B 42B - STYLE 4395 Turkey Boxers (Zara Man) in Lapaz - Clothing, Stephen Kissi
Turkey Boxers (Zara Man) in Lapaz - Clothing, Stephen Kissi The Essential Kit of menstrual underwear - Mme L'Ovary
The Essential Kit of menstrual underwear - Mme L'Ovary Matilda Jane Size 2 Family Heirloom Dress Once Upon A Time Joanna Gaines NWT
Matilda Jane Size 2 Family Heirloom Dress Once Upon A Time Joanna Gaines NWT Mudd Juniors Flared Leggings SZ XS Boho Hippie Style Stretchy Pants
Mudd Juniors Flared Leggings SZ XS Boho Hippie Style Stretchy Pants Arracadas de Oro 14K diseño liso grandes - Joyería Ruben's Compra
Arracadas de Oro 14K diseño liso grandes - Joyería Ruben's Compra