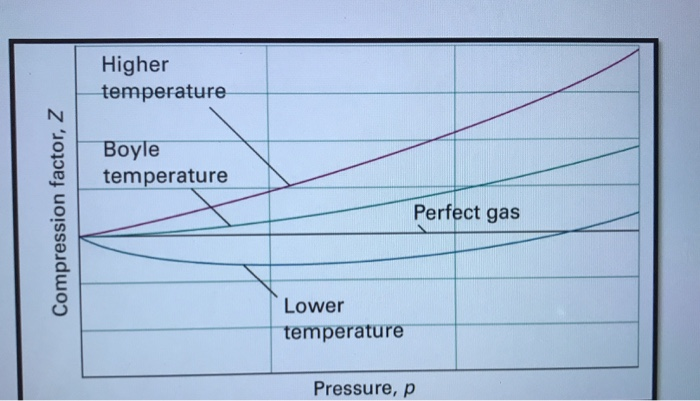
UNUB At Boyle temperature, the value of compressi factor Z has a value of one over a wide range of pressure. This is due to the fact that in the van der
4.6 (528) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion
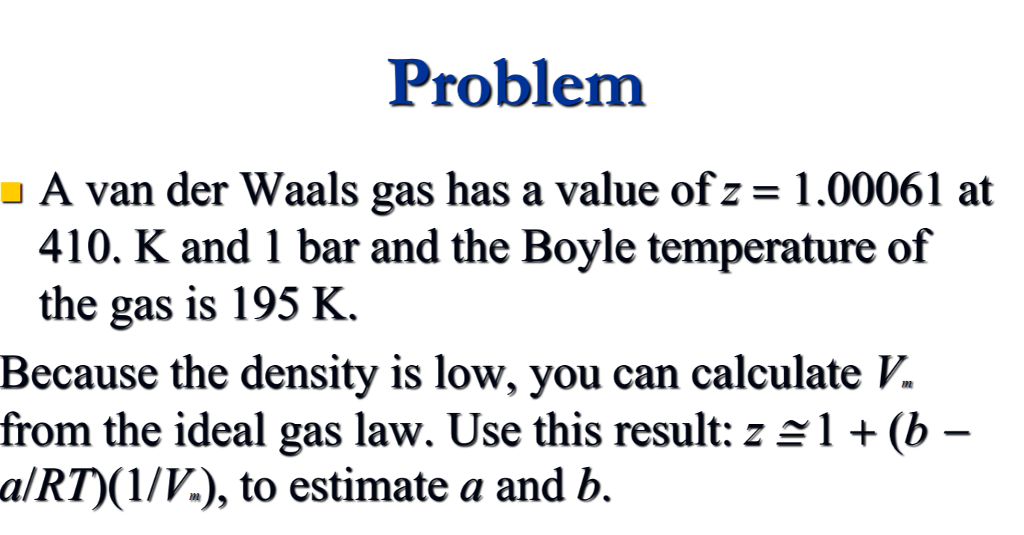
Solved Problem a A van der Waals gas has a value of

At Boyle's temperature, the value of compressibility factor Z = PV

ReasonAll the gases tend to approach a value Z=1, when the

qph.cf2.quoracdn.net/main-thumb-137510504-200-syqq

Determine Compressibility of Gases


economy Archives - Brazilian-American Chamber of Commerce
Question #b3655

3.2 Real gas and compressibility factor – Introduction to

Respostas - Físico-Química (Vol.1) - Atkins PDF
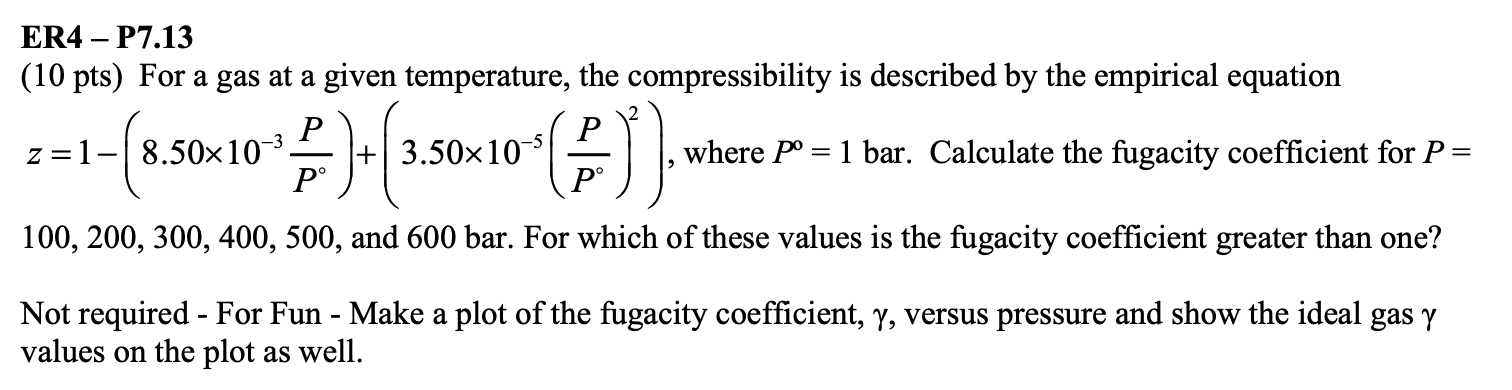
Solved ER4 - P7.13 (10 pts) For a gas at a given

STUDY CFT/EFSA/FEEDAP/2005/01
Solved 6. (a) Discuss the significance of the Boyle
Solved The virial expansion of the compression factor (Z)
PPT - Real gases PowerPoint Presentation, free download - ID:3959491
 Women's Sleepwear - Pajamas, Robes, & Nightgowns
Women's Sleepwear - Pajamas, Robes, & Nightgowns SKIMS SLEEPOVER LACE CAMI AND TAP SHORT SET
SKIMS SLEEPOVER LACE CAMI AND TAP SHORT SET MAGCOMSEN Women's Fleece Lined Waterproof Insulated Softshell Pants Outdoor Snow Ski Pants Winter Warm Hiking Pants Army Green XS : Clothing, Shoes & Jewelry
MAGCOMSEN Women's Fleece Lined Waterproof Insulated Softshell Pants Outdoor Snow Ski Pants Winter Warm Hiking Pants Army Green XS : Clothing, Shoes & Jewelry No Boundaries Juniors Plus Size Flare Pants, 2-Pack, Sizes 1X-4X
No Boundaries Juniors Plus Size Flare Pants, 2-Pack, Sizes 1X-4X Dark maroon coloured taffeta silk embridered work lehenga choli
Dark maroon coloured taffeta silk embridered work lehenga choli Round Capri Brushed Gold Cup Drawer Pull, Ring Pull or Cabinet
Round Capri Brushed Gold Cup Drawer Pull, Ring Pull or Cabinet