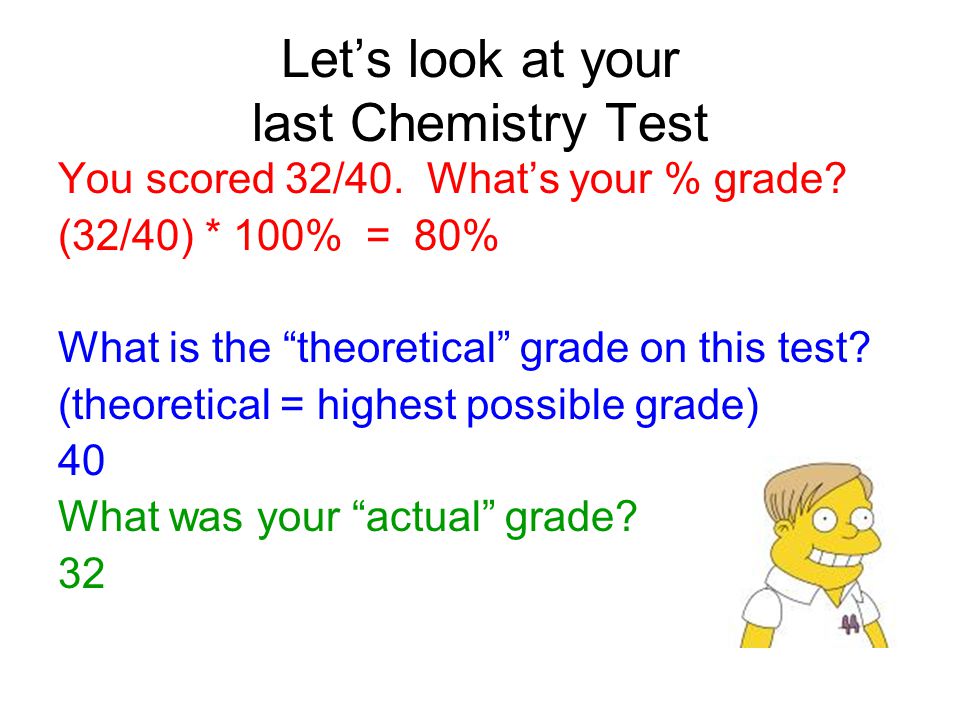
Percentage Yield of a Chemical Reaction. Let's look at your last Chemistry Test You scored 32/40. What's your % grade? (32/40) * 100% = 80% What is the. - ppt download
4.5 (619) In stock
What has this to do with Chemistry? Theoretical yield of a chemical reaction is predicted by stoichiometry. The amount of product obtained by the chemist is the actual yield.
Percentage Yield of a Chemical Reaction
What’s your % grade. (32/40) * 100% = 80% What is the theoretical grade on this test. (theoretical = highest possible grade) 40 What was your actual grade. 32.
Theoretical yield of a chemical reaction is predicted by stoichiometry. The amount of product obtained by the chemist is the actual yield..
Actual yields are often less than theoretical yields due to competing (side) reactions loss of product due to poor lab technique chemical equilibrium (See y’all next year!) impure reactants
Actual yields can also be greater than theoretical yields due to an impure or contaminated product a solid product that hasn’t been sufficiently dried
ie. grams/grams; mol/mol, etc.
in units of grams. 2. Calculate % yield..
Theoretical yield is 1 mol N 2 (g):2 NH 3 (g) 7.5 g ↓(/28.0 g/mol) 0.27 mol (x 2/1) 0.54 mol ↓x 17.0 g/mol 9.1 g NH 3 is the theoretical yield
% =(actual/theoretical) * 100% = (1.7g/9.1g) * 100% =19% (to two sf) Does this answer make sense
Sample Problem 2 Calcium carbonate, CaCO 3, thermally decomposes to produce CaO and CO 2 according to CaCO 3 (s) CaO(s) + CO 2 (g) If the reaction proceeds with a 92.5% yield, what volume, at SATP, of CO 2 can be expected if 12.4 g CaCO 3 is heated
1 mol CaCO 3 (s):1 mol CO 2 (g) 12.4 g ↓/ g.mol mol (x 1/1) mol ↓ x 24.0 L/mol 2.97 L theoretical ↓ x yield 2.75 L actual yield at SATP
p 262 PP 31 – 33 p 264 PP 34 – 37 Homework there’s more
For example, the reactant you massed is only 70% pure. What will this do to the % yield. Yield will be 70%..
When a 13.9 g sample of impure iron pyrite is heated in the presence of oxygen, O 2, 8.02 g of Fe 2 O 3 is produced according to: 4 FeS 2 (s) + 11 O 2 (g) 2 Fe 2 O 3 (s) + 8 SO 2 (g) What is the % purity of the iron pyrite sample .
% purity= (12.0 g/13.9 g) * 100% = 86.3% is the purity of iron pyrite.
Homework PP #38, 39, 40 on p 269 SR #1 – 4 on p 270 Get started on Ch 7 review problems.

Percent Yield - Video Tutorials & Practice Problems

Percent Yield - Video Tutorials & Practice Problems

How to Calculate Theoretical Yield: 12 Steps (with Pictures)

Applying Percent Yield, Practice Exam 1.2

How to Calculate Percent Yield in Chemistry: 15 Steps

Study Guide Percent Yield WS 2 2021, PDF
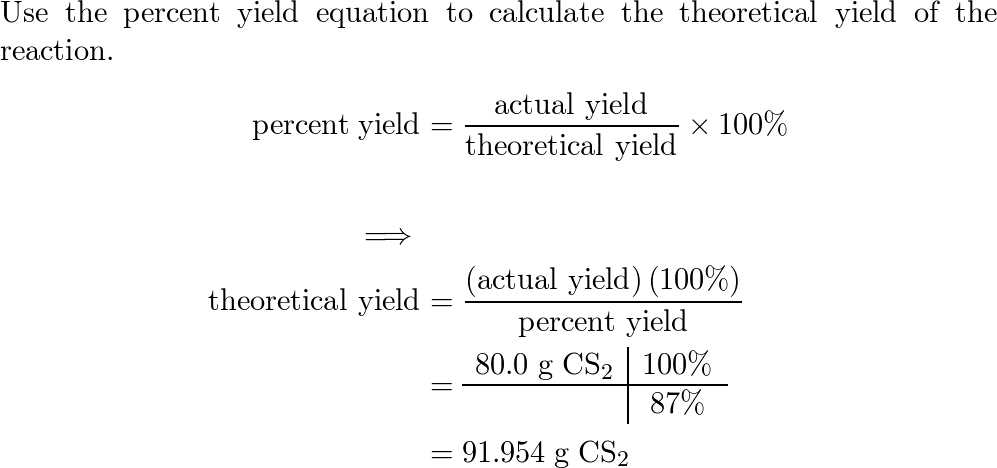
The percentage yield of the following reaction is consistent

How to Calculate the Percent Yield of Chemical Reactions

Percentage Yield of a Chemical Reaction. Let's look at your last

Percentage Yield of a Chemical Reaction. Let's look at your last

Percent Yield in a Chemical Reaction (lab) by Science and The Big
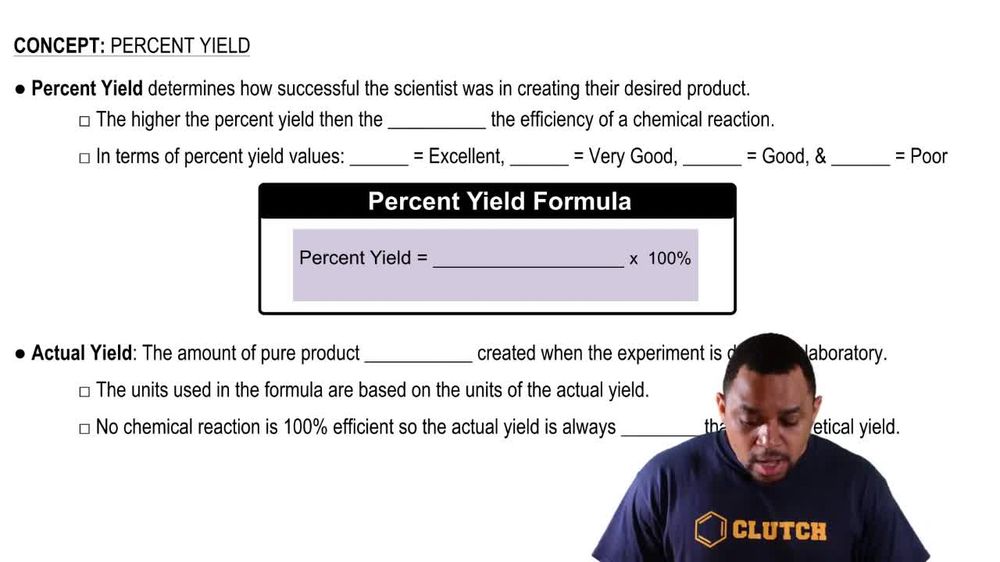
Percent Yield - Video Tutorials & Practice Problems

Answers - Percent Yield Practice PDF

Solved Never assume chemical reactions are balanced and even

Calculate Percent Yield with Ideal Stoichiometry - Practice - 1
40 percent off sale discount composition Vector Image
40% Forty Percent OFF discount sale white red Classic Round Sticker
If 40% of a number is 32, what is 35% of the number? F 8 G 2
 Pacemaker Incision Protector Post Surgery Bra Strap Pad Chest Cushion to Prevent Wound Rubbing for Heart Surgery Recovery Support Pad for Bra Straps Chest Port Cushion Support 2 Pack : : Clothing, Shoes & Accessories
Pacemaker Incision Protector Post Surgery Bra Strap Pad Chest Cushion to Prevent Wound Rubbing for Heart Surgery Recovery Support Pad for Bra Straps Chest Port Cushion Support 2 Pack : : Clothing, Shoes & Accessories Phoebe Price and Ana Braga street sighting in Studio City wearing
Phoebe Price and Ana Braga street sighting in Studio City wearing STACKING RAINBOW SET
STACKING RAINBOW SET Glamorise Magiclift® Full Figure Support Wireless Unlined Full
Glamorise Magiclift® Full Figure Support Wireless Unlined Full Baby Girl Barbie Pink Leopard Blanket, ZHOHAO05
Baby Girl Barbie Pink Leopard Blanket, ZHOHAO05 Optical Cross
Optical Cross