ChemE 260 Equations of State April 4, 2005 Dr. William Baratuci Senior Lecturer Chemical Engineering Department University of Washington TCD 2: E & F CB. - ppt download
4.6 (108) In stock

Advanced Equations of State Compressibility Factor EOS (graphical) Virial EOS Van der Waals EOS Redlich-Kwong EOS Soave-Redlich-Kwong EOS Baratuci ChemE 260 April 4, 2005
William Baratuci Senior Lecturer Chemical Engineering Department University of Washington TCD 2: E & F CB 2: 6 – 8, Supplement.
–When molecules interact very little with each other –At high T and low P –Generally: –Diatomic gases are especially unlikely to interact Baratuci ChemE 260 April 4,
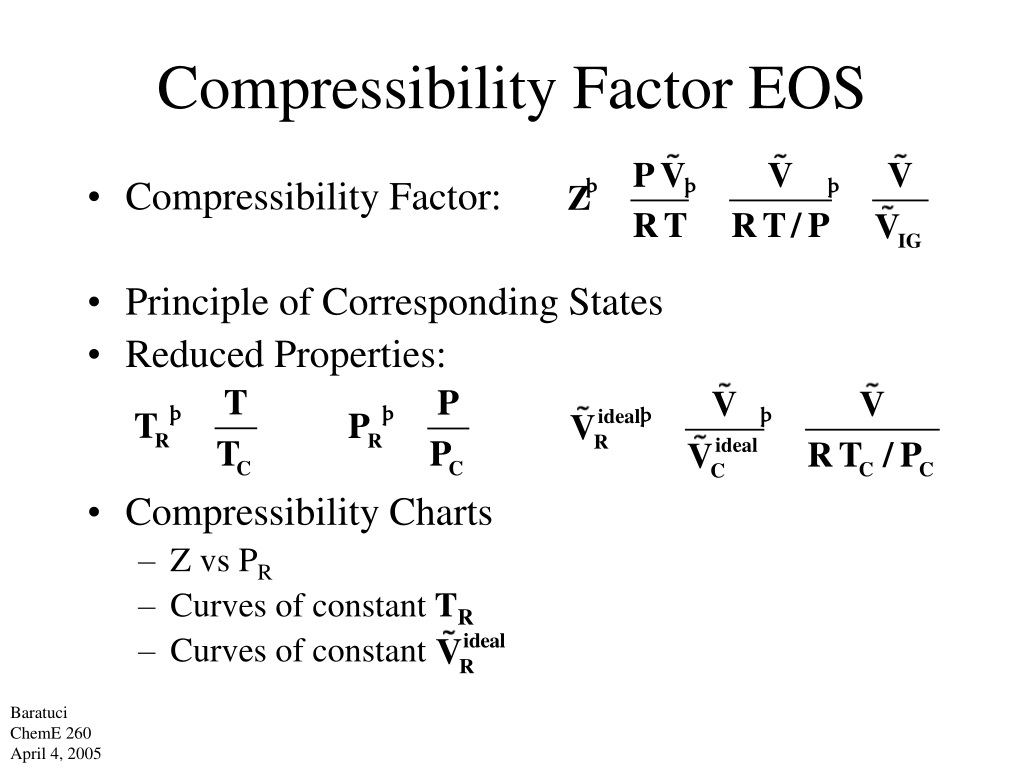
Compressibility Factor EOS Compressibility Factor: Principle of Corresponding States Reduced Properties: Compressibility Charts –Z vs P R –Curves of constant T R –Curves of constant Baratuci ChemE 260 April 4, 2005
Virial EOS Uses a power series expansion to describe deviations of Z from 1, the IG value B, C, D, etc are the Virial constants –functions of T, only –Determined experimentally Truncated Virial EOS: –Estimating B: Baratuci ChemE 260 April 4, 2005
Van der Waals EOS First cubic EOS Constants have physical interpretation Baratuci ChemE 260 April 4, 2005
RK & SRK EOS’s Redlich-Kwong Soave-Redlich-Kwong Baratuci ChemE 260 April 4, 2005
Applications of EOS’s Given any 2 of the 3 variables, determine the value of the unknown Cubic EOS’s and other even more sophisticated EOS’s can be used to… –predict properties of liquids –Estimate molar internal energies, enthalpies and entropies of gases and liquids –In this way, sophisticated EOS’s are used to generate the Thermodynamic Data Tables that we use Baratuci ChemE 260 April 4, 2005
After that… –Chapter 3 – Heat Effects Internal Energy and Enthalpy Using the NIST Webbook Baratuci ChemE 260 April 4,
The Ideal Gas EOS The Virial EOS The van der Waal EOS The Soave-Redlich-Kwong EOS The Compressibility Factor EOS The Steam Tables Baratuci ChemE 260 April 4,
Example #1 – Answers Ideal Gas: Ans.: P = kPa Virial: Ans.: P = kPa van der Waal:Ans.: P = kPa SRK:Ans.: P = kPa Z-Factor:Ans.: P = kPa P = kPa Steam Tables:Ans.: P = kPa Baratuci ChemE 260 April 4, 2005

ChemE 260 Phase Equilibrium and Thermodynamic Data Tables April 1

ChemE 260 Phase Equilibrium and Thermodynamic Data Tables April 1

PPT - Non-ideal Equations of State PowerPoint Presentation, free
PPT - ChemE 260 Foundation of Thermodynamics PowerPoint

ChemE 260 Reversibility and Irreversibility April 27, 2005 Dr

ChemE 260 Equations of State April 4, 2005 Dr. William Baratuci

PPT - Pressure-Volume Equations of State PowerPoint Presentation

ChemE 260 Equations of State April 4, 2005 Dr. William Baratuci

ChemE 260 Phase Equilibrium and Thermodynamic Data Tables April 1
PPT - ChemE 260 Equations of State PowerPoint Presentation, free

ChemE 260 Conservation of Mass & Energy, Steady-State Processes

PPT - Non-ideal Equations of State PowerPoint Presentation, free
Class Notes on Compressibility of a Real Gas, CH 417, Study notes Physical Chemistry
At Critical Temperature,pressure and volume . The compressibility
Procedure calculates base gas compressibility factors
 Women Open Bust Full Body Shaper Seamless Slim Shapewear Tummy Control Bodysuit Briefer Slimmer Corset
Women Open Bust Full Body Shaper Seamless Slim Shapewear Tummy Control Bodysuit Briefer Slimmer Corset- Cup Cozy Pillow
 TLC Monogram Leggings in Black Raspberry
TLC Monogram Leggings in Black Raspberry BALEAF Yoga Workout Capris for Women Lounge Flare Pants Casual Work Bootcut with Side Pockets - 21
BALEAF Yoga Workout Capris for Women Lounge Flare Pants Casual Work Bootcut with Side Pockets - 21 Race Back Rhinestone Bra Straps Diamate Shoulder Dress Straps Sexy
Race Back Rhinestone Bra Straps Diamate Shoulder Dress Straps Sexy) Buy CozyCare 15pcs XL Sanitary Napkins for Women with Heavy Flow Super Absorbent Sanitary Pads for Women Online at Best Prices in India - JioMart.
Buy CozyCare 15pcs XL Sanitary Napkins for Women with Heavy Flow Super Absorbent Sanitary Pads for Women Online at Best Prices in India - JioMart.
