At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry
4.8 (474) In stock

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior. Their equation of state is given as P=RTV−b at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?
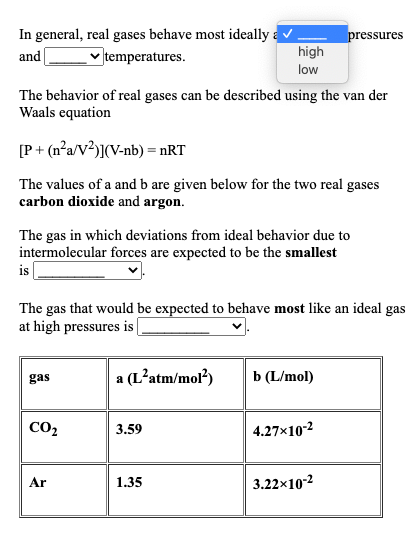
Solved In general, real gases behave most ideally a

Answered: as an Ideal gas at temperatures above…

Ideal gas law assignment 1 - CHEM 1050 Ideal Gas Law Problems Name: An - Studocu

Assume we have ideal gas behavior. We have the

Chaudhery Mustansar Hussain - Handbook of Environmental Materials Management-Springer International Publishing (2019), PDF, Environmental Remediation

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour.

Q.6 At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given RT as p = V-b T.

Identity of Thermodynamic Temperature Scale with the Perfect Gas

Solved In general, real gases behave most ideally at

Deviation From Ideal Gas Behavior - Study Material for IIT JEE

Behaviour of Real Gases, PDF, Gases
Solved 9 Compression factor Z Use the van-der-Waals equation
Derive an expression for the compression factor of a gas tha
The Compression Factor, Z, and Real Gases - What you NEED to Know!
Solved 1. Consider the following gas at a given temperature.
53 pts!! The function f(x)= 7^x+1 is transformed to function g





