The compressibility factor a real gas high pressure is:-1 - frac
5 (251) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor for a real gas at high pressure is
Click here👆to get an answer to your question ✍️ The compressibility factor a real gas high pressure is-1 - frac-Pb- -RT-1 - frac -RT- -Pb-11 - frac -Pb- -RT
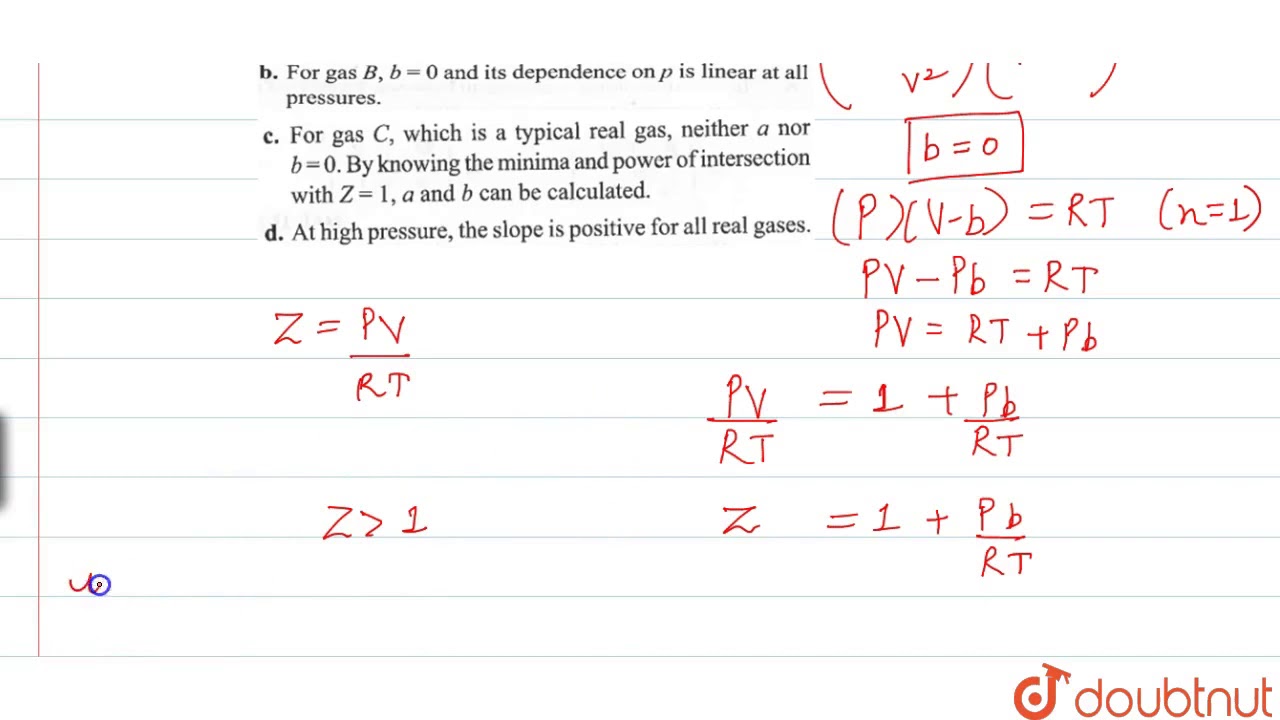
The given graph represents the variations of compressibility factor `Z=PV//nRT` vs `

Gas compressibility factor Z: Ideal gas vs Real gas

Real Gases and Compressibility Factor

At moderate pressure, the compressibility factor a particular gas is given by: {text{Z = 1 + 0}}{text{.3p - }}frac{{160p}}{T} (p in bar and T in kelvin). what is the Boyle's temperature of

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))

physical chemistry - Pressure vs volume plot for real gas and ideal gas - Chemistry Stack Exchange

Van der Waals Equation, Definition & Examples - Lesson

Description of real gases: Compression factor

Gas compressibility factor Z: Ideal gas vs Real gas
Compressibility Factor of Carbon Dioxide - Maple Application Center
1.7: Connecting the van der Waals and the viral equations: the
Developing a Thermodynamical Method for Prediction of Activity
 gymshark asymmetric sports bra in blue steel indigo
gymshark asymmetric sports bra in blue steel indigo Most Comfortable Leather Inside the Waistband Holster - Designed
Most Comfortable Leather Inside the Waistband Holster - Designed SELETA - Blue Saree Shapewear Cotton Women's Shaping Bottoms ( Pack of 1 ) - Buy SELETA - Blue Saree Shapewear Cotton Women's Shaping Bottoms ( Pack of 1 ) Online at Best Prices in India on Snapdeal
SELETA - Blue Saree Shapewear Cotton Women's Shaping Bottoms ( Pack of 1 ) - Buy SELETA - Blue Saree Shapewear Cotton Women's Shaping Bottoms ( Pack of 1 ) Online at Best Prices in India on Snapdeal 6 Sports Bras For Full Busted Women
6 Sports Bras For Full Busted Women Backless crop Top Backless crop top, Backless top outfit, Crop top outfits
Backless crop Top Backless crop top, Backless top outfit, Crop top outfits Alcea rosea 'Double Pink' Double Pink Hollyhock Hollyhocks flowers, Hollyhock, Beautiful flowers
Alcea rosea 'Double Pink' Double Pink Hollyhock Hollyhocks flowers, Hollyhock, Beautiful flowers