Compressibility factor (Z) for a van der Waals real gas at
4.6 (327) In stock

Share your videos with friends, family and the world
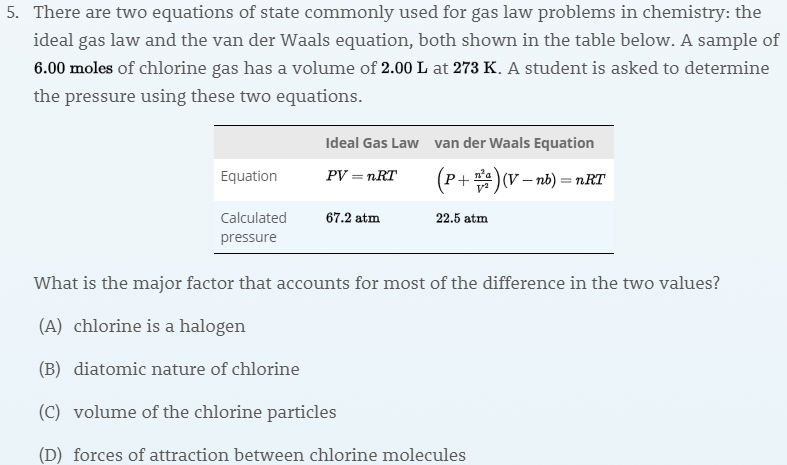
What is the major factor that accounts for most of the difference in these two values of pressure (ideal gas law vs. van der Waals equation)?
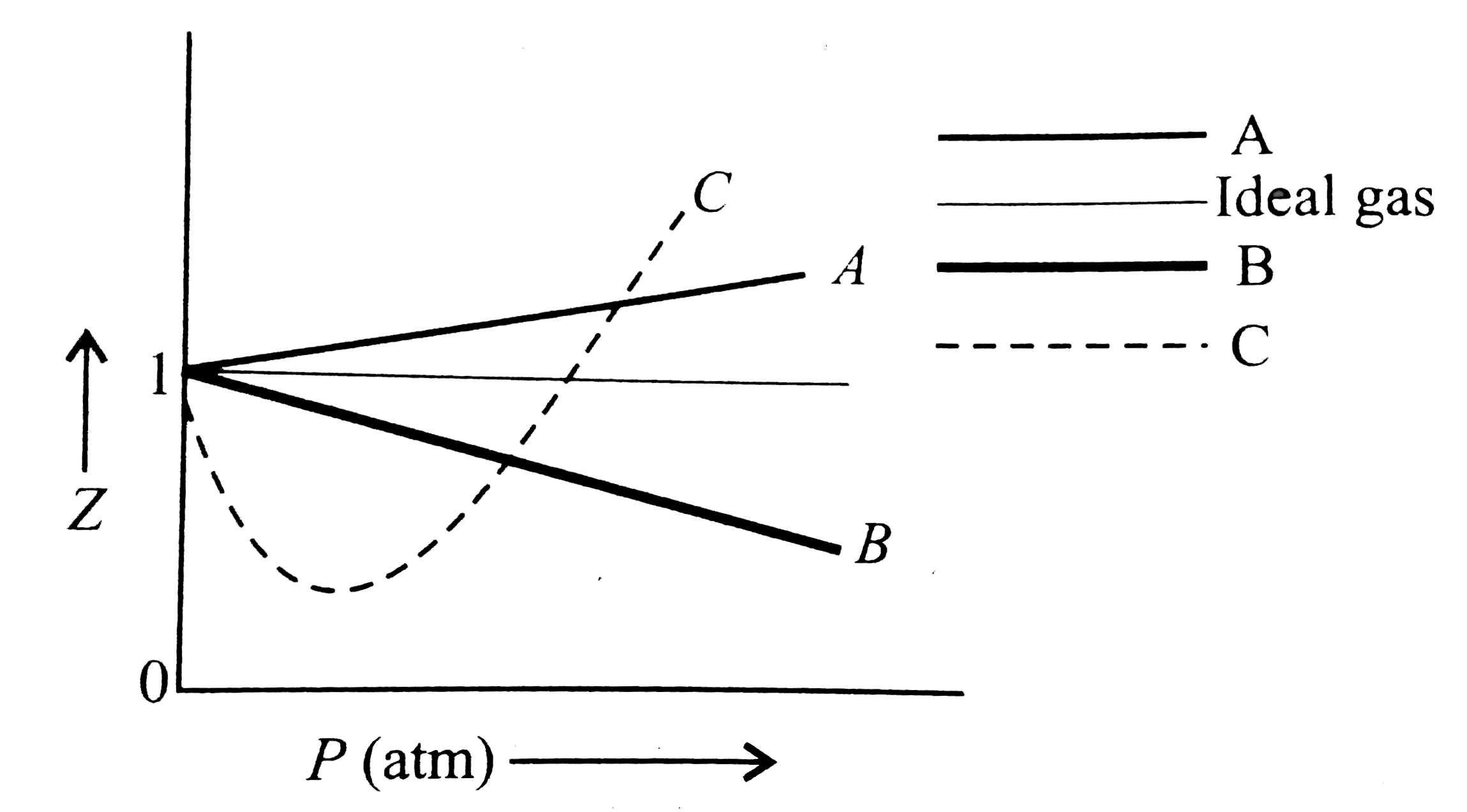
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

Maxwell's speed distribution curve

6.3: Van der Waals and Other Gases - Physics LibreTexts

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

SOLUTION: State of matter gases liquids and solids - Studypool

Punjabi] For a real gas (mol.mass =60) if density at critical point i

Compressibility Factor Z Important Concepts and Tips for JEE Main

Deviation From Ideal Gas Behavior - Study Material for IIT JEE
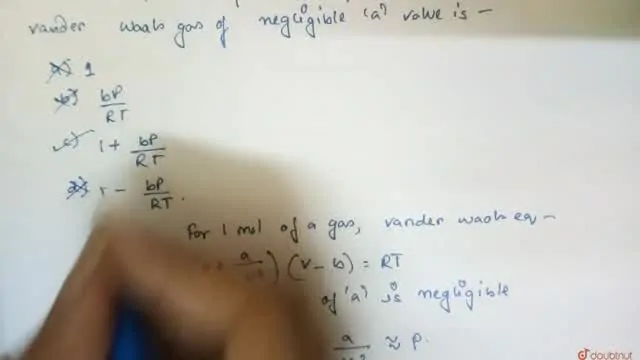
Bengali] The compresibility factor (Z) of one mole of a van der waals
Compressibility factor (gases) - Knowino
Compressibility Factor Z Important Concepts and Tips for JEE Main
- Calvin Klein Underwear MODERN COTTON STRETCH HOLIDAY TRUNK 5 PACK Multi
 Time and Tru Women's High Rise Jeggings, 29 Inseam, Sizes XS-3XL
Time and Tru Women's High Rise Jeggings, 29 Inseam, Sizes XS-3XL Women's Panties Cotton Underwear Girls Briefs Plus Size Lingeries
Women's Panties Cotton Underwear Girls Briefs Plus Size Lingeries Women's Posture Correction Bra Chest Contouring Band Chest Support Back Support
Women's Posture Correction Bra Chest Contouring Band Chest Support Back Support- ELLE Seamless Rib Lace Bralette, Blue Spruce, S
 Get Thin Arms in Only 5 Minutes
Get Thin Arms in Only 5 Minutes

