The role of the compressibility factor Z in describing the volumetric behavior of gases
4.7 (449) In stock

In this post I will give a recapitulation of the role the compressibilty factor Z plays in the volumetric behavior of gases. The purpose of this post is also to give some background to the first post of June , 2013 describing a compact, explicit equation for the vapor compressibility factor Z in the sub-critical…
Membranes, Free Full-Text

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

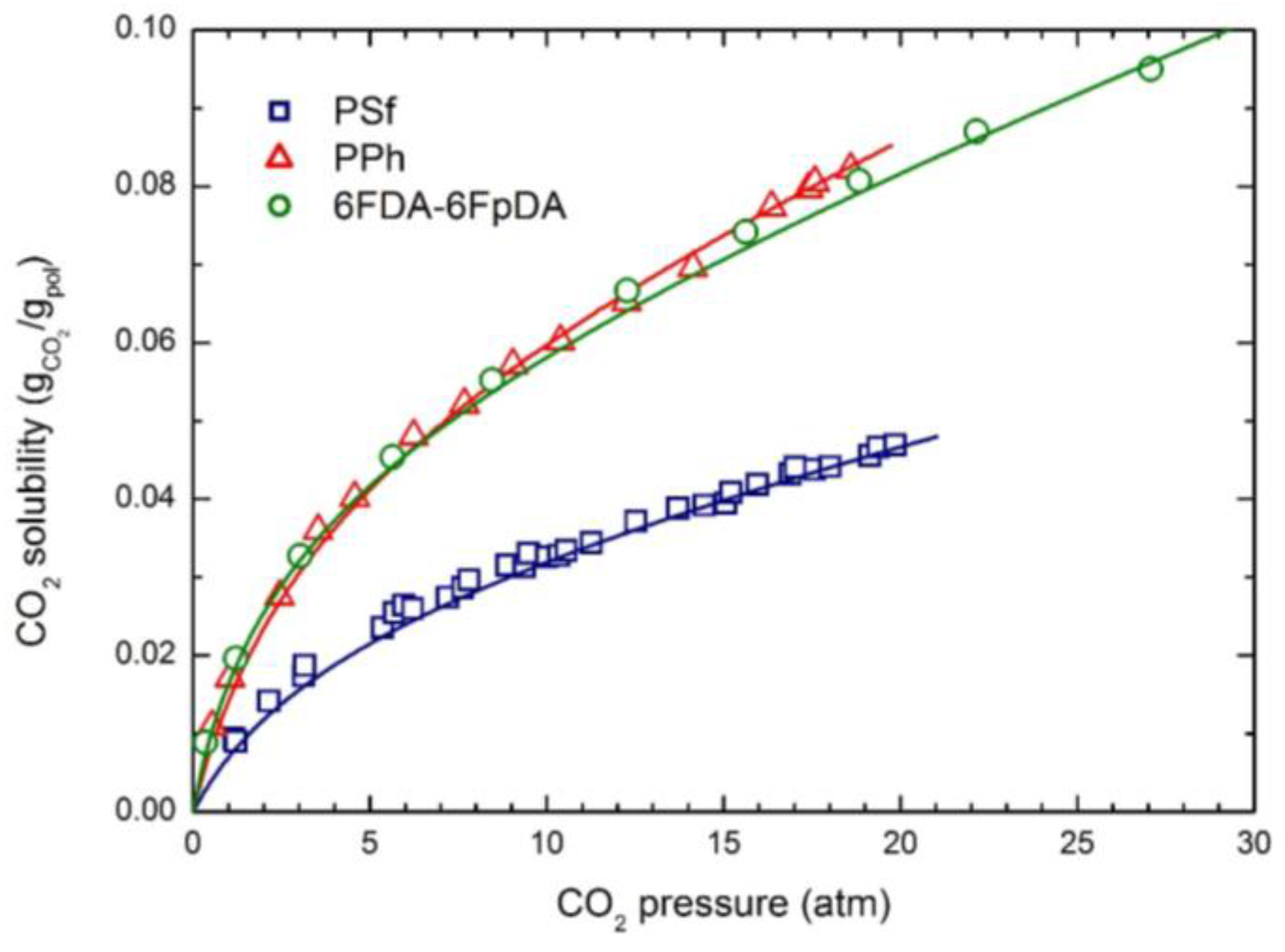
A thermodynamic study on relationship between gas separation properties and microstructure of polyurethane membranes

Objectives_template

Real Gas Behavior The Compression Factor (Z) [Example #2]

Gas Compressibility - an overview

Gas compressibility factor Z: Ideal gas vs Real gas

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1

compressibility - Pasifik Blower

Gas compressibility factor Z: Ideal gas vs Real gas

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Real Gases vs Ideal Gases & the Compressibility Factor
In the following compressibility factor (Z) vs. pressure graph 300
PVT Data from Compressibility Factor Table
The compressibility factor Z a low-pressure range of all gases
Compressibility factor of water
Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor
 Ladies sze 14 vintage style cotton interlock eyelet knickers panties brief White
Ladies sze 14 vintage style cotton interlock eyelet knickers panties brief White This is Magic hair cloe and Yasmin I think! Just got Yasmin in the mail : r/ Bratz
This is Magic hair cloe and Yasmin I think! Just got Yasmin in the mail : r/ Bratz 12 Season Color Analysis, Indigo Tones
12 Season Color Analysis, Indigo Tones knix, Pants & Jumpsuits
knix, Pants & Jumpsuits Colombia Jeans High-Waist Butt-Lifting Jeans
Colombia Jeans High-Waist Butt-Lifting Jeans Body contour seamless tanga brief [Forest Green]
Body contour seamless tanga brief [Forest Green]